Unsere Forschung
We are interested in the immunological mechanisms which govern homeostasis at mucosal surfaces and the pathways contributing to disruption of homeostasis under conditions of inflammation and tumor development. Our work focuses on the intestine and the liver and associated inflammatory disorders such as inflammatory bowel disease as well as malignant diseases such as colorectal cancer and hepatocellular carcinoma. We are pursuing a translational research strategy combining work in genetically modified mice with studies on human tissues and cells. Specific current research topics are:
Immunological alterations and defects in epithelial regeneration in the pathogenesis of inflammatory bowel disease
Inflammatory bowel disease (IBD) is a group of disorders characterized by chronic intestinal inflammation. In the vast majority of IBD patients, intestinal inflammation occurs through a complex and incompletely understood interplay of genetic and environmental factors. However, we and others have identified monogenic forms of IBD, in which individual genetic defects give rise to intestinal inflammation with high penetrance (Zeissig Y, Gut 2015; Zeissig S, Gut 2015). These monogenic forms of IBD have provided tremendous insight into the pathways which govern control of intestinal homeostasis and which lead to chronic intestinal inflammation and damage if disrupted. Moreover, this work has led to novel personalized strategies for the treatment of IBD. Current work focuses on host-microbial interactions in the pathogenesis of IBD and defects in the intestinal epithelium, which are associated with altered regeneration and differentiation as well as chronic intestinal inflammation (Južnić et al., Gut 2020).
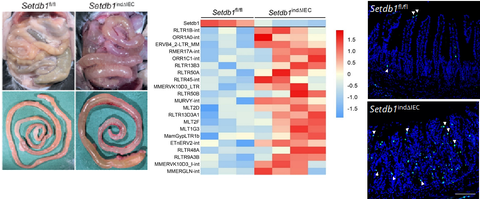
Intestinal inflammation (left) resulting from intestinal epithelial deletion of the H3K9 methyl transferase Setdb1, which leads to replication of endogenous retroviruses (middle) and intestinal damage (right). Click image to enhance.
Interactions between lipid metabolism, inflammation, and carcinogenesis
Cellular metabolism has major roles in the regulation of immunity, inflammation, and cancer. We are investigating this crosstalk through the lens of lipid metabolism and its contribution to intestinal and hepatic inflammation and carcinogenesis. We could show that lipid metabolism controls immunological homeostasis in the liver (Zeissig et al., Nat. Med. 2012; Zeissig et al., Proc Natl Acad Sci USA 2017) and the intestine (Olszak et al., Nature 2014; Olszak et al. Science 2012) and that metabolic alterations are associated with defects in NKT cell-dependent immunity (Zeissig et al., J Clin Invest 2010; Melum et al., Nat Immunol 2019). Current work focuses on alterations in lipid metabolism in colorectal cancer and is based on lipidomics studies (with the Shevchenko group at MPI-CBG) in combination with functional studies in mice.
Microbial and immunological regulation of cancer development in the intestine and the liver
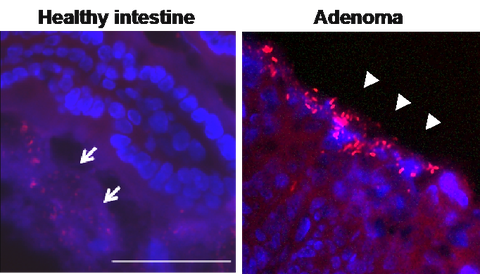
Chronic inflammation is a risk factor for the development of cancer. Examples for such associations in the gastrointestinal tract include the links between inflammatory bowel disease (IBD) and colorectal cancer (CRC), Helicobacter pylori infection and gastric cancer, Hepatitis B and C virus infections and hepatocellular cancer as well as non-alcoholic fatty liver disease and hepatocellular cancer. In many of these examples, inflammation is clinically inapparent, restricted to the respective tissue and dependent on microbial organisms. In line with these principles, we could recently demonstrate that colorectal adenomas and carcinomas are associated with disruption of the intestinal barrier and translocation of bacteria into host tissue (Peuker et al., Nat. Med. 2016). This is associated with the activation of inflammatory pathways and promotes tumor growth. Based on this work, we are currently pursuing studies to delineate pathways of microbial and immunological regulation of colorectal cancer development. Furthermore, we are studying whether mechanism of barrier disruption and microbiota-dependent tumor development are also active in the liver and promote hepatocellular carcinoma development in the context of non-alcoholic fatty liver disease.
Bacterial infiltration (red) into host tissue in intestinal adenomas (Peuker et al., Nat. Med. 2016). Click image to enhance.