06.12.2022
Europäischen Kommission genehmigt: "New strategies for advanced material-based technologies in health applications"
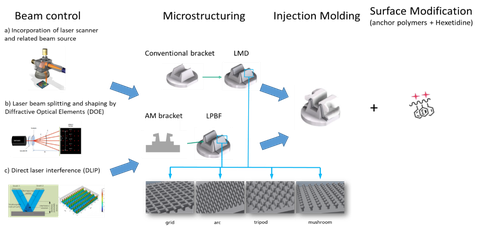
SMILE-" baut auf den Ergebnissen des Vorgängerprojektes "3D Mikrodent" auf
In diesem Jahr wurden zwei Forschungsprojekte von der Europäischen Kommission im Rahmen eines M-ERA.NET Call 2021 zum Thema "New strategies for advanced material-based technologies in health applications" genehmigt.
Beide Projekte: "-SMILE-" und "Mem4BoTiReg" wurden vom Lehrstuhl für Kieferorthopädie an der Medizinischen Fakultät Carl Gustav Carus der TU Dresden eingereicht. Die Laufzeit beider Projekte beträgt 3 Jahre und die Gesamtfinanzierung beider Projekte beläuft sich auf 2,25 Millionen Euro.
SMILE- Oberflächenbeschichtung und MIkrostrukturierung für zusammengesetzte funktionsfähige Biomaterialien in der Zahnmedizin
Zusammenfassung des Projekts
Aufgrund des wachsenden Interesses an ästhetischen Lösungen bei einer zunehmenden Zahl junger Erwachsener und erwachsener Patienten besteht ein Bedarf an neuen Materialien für die festsitzende kieferorthopädische Behandlung, insbesondere, weil die Aligner-Systeme nicht für schwierige Zahnbewegungen verwendet werden können. Eine alternative ästhetische Option ist die Verwendung von festsitzenden Lingualsystemen, die sehr teuer und in manchen Fällen nicht geeignet sind, oder die Verwendung von Keramikspangen, die jedoch im Unterkiefer wegen des Schmelzabriebs nicht eingesetzt werden können. Um dieses Problem zu lösen, wird ein wettbewerbsfähiges Produkt benötigt, das sowohl die mechanischen als auch die ästhetischen Anforderungen erfüllt und gleichzeitig die Nachteile von Multimaterialien durch eine antimikrobielle Substanz zur Verringerung des Kariesrisikos ausgleicht.
Ziel des aktuellen Projekts ist daher die Entwicklung von Metall-Polymer-Hybridbauteilen für die zahnmedizinische Anwendung, wobei zunächst die Materialgrenzfläche durch additiv hergestellte Mikrotopologie (Makro- und Mikrostrukturierung) und in einem zweiten Schritt durch verbesserte Polymerinfiltration verbessert wird. Zusätzlich wird eine aktive antimikrobielle Substanz zur Reduzierung der oralen Biofilmbildung die Komposit-Materialoberfläche funktionalisieren. Obwohl in der Zahnmedizin verschiedene Anwendungsszenarien denkbar sind, befasst sich dieses Projekt mit dem Bereich der Kieferorthopädie, insbesondere mit der Herstellung moderner Zahnbrackets auf der Grundlage dieses funktionalisierten Multimaterials.
Die spezifische Innovation liegt in der Verbesserung der Metall-Polymer-Grenzflächenverriegelung, die durch additive Fertigung und Oberflächenfunktionalisierung mit einer aktiven antimikrobiellen Substanz erzeugt wird. Bislang ist für die ästhetische kieferorthopädische Behandlung kein Material auf dem Markt, das diese Funktionen kombiniert.
Um evidenzbasierte Ergebnisse zu erhalten, wird ein komplexes statistisches Designverfahren angewandt, das nicht nur In-vitro- und In-vivo-Tests der mechanischen und biologischen Eigenschaften dieser neuen Komposit-Zahnspange umfasst, sondern auch präklinische und klinische Studien. Es wird ein striktes Datenmanagement eingehalten, das sich an die Open-Source-Agenda der Europäischen Kommission hält, außer in den Fällen, in denen dies die Kommerzialisierungsaussichten des Konsortiums beeinträchtigt, und die Daten werden durch die Anwendung moderner rechnerischer und statistischer Techniken erforscht. Dies wird vermarktbar sein, um ein Produkt als Benchmark im Industriedesign optimal herzustellen, für das die Standardmetallproduktionsgeschwindigkeit und -kosten zur Orientierung herangezogen werden können.
"-SMILE-" wird einen solchen dynamischen Austausch zwischen Kollegen aus Deutschland - TU Dresden, Medizinische Fakultät Carl Gustav Carus (Lehrstuhl für Kieferorthopädie) und Fraunhofer IWS, Polen - Technische Universität Bialystok und der rumänischen Universität POLITEHNICA Bukarest - etablieren, um die Anforderungen an eine marktfähige Prozesskette im medizinischen Bereich zu erfüllen und ausreichende Daten zu liefern, um die Evidenz dieses neuen Multi-Material-basierten Geräts in diesem Bereich zu demonstrieren.
Das Projekt "-SMILE-" baut auf den Ergebnissen des Vorgängerprojektes "3D Mikrodent" (https://agent3d.de/mikrodent) auf und wird von PD Dr. med. dent. Ute Botzenhart von der TU Dresden in Abstimmung mit allen Partnern koordiniert. PD Dr. U. Botzenhart gehört bereits zu den Ideengebern des Vorgängerprojektes, das Teil des 3D-Agent-Konsortiums war, und wird gemeinsam mit hochqualifizierten Partnern das Thema auf internationaler Ebene weiterverfolgen.
"Mem4BoTiReg - Funktionalisierte Membran für (4) Knochen- und Geweberegeneration" im Rahmen des M-ERA.Net Calls 2021
Zusammenfassung des Projekts
Knochendefekte unterschiedlicher Größe, die durch Traumata, Fehlbildungen, pathologische Degenerationen, Gaumenspalten oder iatrogene Einflüsse entstanden sind, erfordern häufig rekonstruktive Knochenaufbaumaßnahmen. Die Behandlungen umfassen die Verwendung einer Vielzahl von chirurgischen Ansätzen, Knochentransplantaten und Barrieremembranen.
Eine der besten Methoden zur Realisierung der Knochenregeneration im Bereich des Alveolarkamms ist die Anwendung von Techniken der gesteuerten Knochen-/Geweberegeneration (GBR/GTR). GTR ist ein chirurgisches Verfahren, bei dem sowohl nicht abbaubare als auch biologisch abbaubare Barrieremembranen verwendet werden, um das Wachstum neuen Gewebes an Stellen zu steuern, an denen das Volumen oder die Größe des Gewebes für eine ordnungsgemäße Funktion, Ästhetik oder prothetische Versorgung nicht ausreicht. Während sich GTR mit Weichgewebe befasst, konzentriert sich GBR auf die Trennung von Knochen und Bindegewebe, um dessen schnelles Einwachsen zu verhindern. Biologisch abbaubare Kollagenmembranen werden in den meisten Fällen bevorzugt, um einen zusätzlichen chirurgischen Eingriff zur Entfernung der nicht abbaubaren Membranen zu vermeiden. Herkömmliche Kollagenmembranen sind jedoch in der Regel instabil und können in den Defekt kollabieren. Darüber hinaus erfordern mehrdimensionale Knochendefekte die Anwendung volumenstabiler und tragfähiger Membranen, was derzeit nur durch nicht abbaubare synthetische Materialien gewährleistet ist. Daher ist die klinische Notwendigkeit, eine neue Generation volumenstabiler Barrieremembranen zu entwickeln, die Biokompatibilität, strukturelle Stabilität und patientenspezifische Form mit biologisch abbaubaren Eigenschaften kombinieren, von großem Interesse. Ein wesentlicher Vorteil solcher Membranen wäre neben ihren verbesserten mechanischen Eigenschaften auch eine aktive und spezifisch fördernde Funktion in den Regenerationsprozessen von Knochen und Weichgewebe sowie die exakte und stabile Anpassung in den Defektbereichen.
Das vorliegende Projekt hat zum Ziel, eine neue volumenstabile Barrieremembran aus dem biologisch abbaubaren Polymer Polymilchsäure (PLA), funktionalisiert mit bioaktiven Nanopartikeln und dem Wachstumsfaktor Bone Morphogenetic Protein 2 (BMP-2), zu entwickeln, zu charakterisieren und in vitro und in vivo zu untersuchen. Auf dem europäischen Markt gibt es noch keine zugelassene enganliegende Membran, die mit Wachstumsfaktoren angereichert ist, obwohl diese als wirksam für die Knochenregeneration gelten.
Die Originalität der neuen Membranen liegt in der personalisierten Form, den verbesserten mechanischen Eigenschaften für eine bessere Anwendung, der definierten biologischen Abbaubarkeit und der gezielten therapeutischen Wirkung. Für das computergestützte Design der 3D-Membranen werden beispielhaft Cone Beam Computed Topographical-Bilder von Patienten mit kleinen Knochendefekten verwendet. Der Vorteil dieses Ansatzes liegt zum einen in der präzisen Anpassung der Membran an den Knochendefekt und die Zähne zur Maximierung der Stabilität und Abdichtung und zum anderen in der Unterstützung des Einwachsens von Knochenzellen in die Defektstelle und der beschleunigten Vaskularisierung. Unterschiedliche Strukturen und Porengrößen, die für das Einwachsen von Zellen entscheidend sind, bieten zwei Herstellungsverfahren: Elektrospinnen und schnelles, tomographisches, volumetrisches 3D-Drucken, die in diesem Projekt verwendet werden, um die am besten geeignete Herstellungsmethode einschließlich eines einfachen Sterilisationsprozesses der Produkte als zwingende Voraussetzung für den klinischen Einsatz zu bewerten. Durchführung von präklinischen In-vivo-Implantationstests zur Bewertung des Verhaltens von Barrieremembranen in einem Tiermodell, um ihre tatsächliche biomechanische Integrität, die Eigenschaften des biologischen Abbaus und der Vaskularisierung sowie die Regenerationsprozesse von Knochen und Bindegewebe zu bestimmen.
Das Projektkonsortium besteht aus Partnern aus drei verschiedenen Ländern. Der Standort von zwei Partnern in Deutschland ist Dresden. Der Schwerpunkt der ausländischen Partner liegt in São Paulo (Brasilien) und Dębica (Polen). Die Forschungsgruppen aller vier Partner umfassen Wissenschaftler mit überdurchschnittlichen Kenntnissen auf dem Gebiet der regenerativen Medizin, innovativer Techniken zur Herstellung von Medizinprodukten, deren Funktionalisierung und Charakterisierung. Eine große Gruppe von Chemikern, Biochemikern, Ärzten, Zahnärzten, Biologen, Veterinärmedizinern und Vertretern kleiner und mittlerer Unternehmen wird sich an diesem Projekt beteiligen.
Die Technische Universität Dresden bezieht sich auf zwei unabhängige Einheiten der Medizinischen Fakultät: Lehrstuhl für Kieferorthopädie und Zentrum für Translationale Knochen-, Gelenk- und Weichgewebeforschung (Leitung Prof. Dr. rer. nat. Michael Gelinsky).
Der Projektkoordinator ist PD Dr. rer. med., Dr. med.dent. Tomasz Gredes.
