Projects
1. Role of hematopoietic stem and progenitor cells in native hematopoiesis
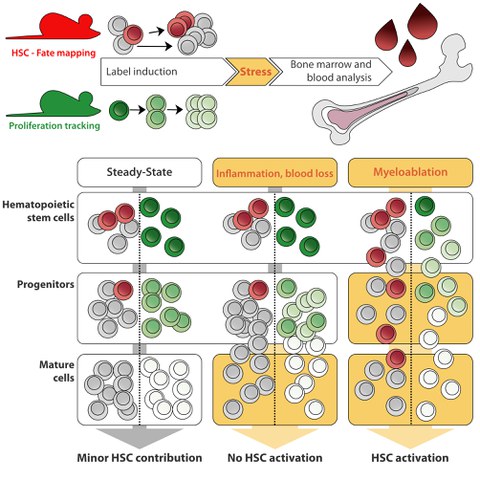
An adult human replenishes approximately 1011 mature blood cells per day, making blood the most regenerative tissue of all. To study the proliferation and differentiation of hematopoietic stem and progenitor cells in their physiological environment, we use state-of-the-art mouse models. For example, we use Cre/loxP-based approaches for selective labeling of HSCs in living animals to follow their contribution to blood cell production. To analyze and model our experimental data, we collaborate with theoretical scientists, which enables quantitative understanding of native hematopoiesis. In collaboration with the lab of Thomas Höfer, DKFZ Heidelberg, we have elucidated an alternative pathway of thrombopoiesis in which HSCs directly differentiate into megakaryocytes, bypassing the classical multipotent progenitor intermediates (Morcos, Li, Munz et al., Nature Communications 2022). In collaboration with the group of Ingmar Glauche, IMB TU Dresden, we were able to show that HSCs in adult mice are predominantly quiescent, but do not undergo a permanent cell cycle arrest (Morcos et al., Journal of Experimental Medicine 2020).
HSCs are thought to be a reserve population residing in a deeply quiescent ("dormant") state, protecting them from metabolic and proliferative stress. According to this concept, HSCs are activated by an increased demand for mature blood cells in situations such as systemic infection or severe blood loss. Frequent exposure of HSCs to such stressors has been shown to result in a gradual attrition of their transplantation potential. Using our fate mapping and proliferation reporter mouse models, we recently investigated how native hematopoiesis responds to systemic inflammation or blood loss (Munz et al., Blood 2023). Contrary to popular belief, we found that these challenges do not robustly stimulate HSC differentiation and proliferation. Only severe disruptive "myeloablative", conditioning, including high-dose ionizing radiation or chemotherapy, requires HSC input.
In ongoing research projects, we are investigating native hematopoiesis following mutations that either reduce genome replication fidelity (Dressel, Natusch et al.; bioRxiv 2022) or predispose to leukemia development. In addition, we have recently started to use mouse models in which inheritable genetic barcodes are introduced into single cells. This exciting technology allows us to follow native hematopoiesis at the clonal level.
The rare contribution of HSCs to adult steady-state and stress hematopoiesis raises the question of the function of these cells. The relevance of HSCs for adult hematopoiesis was further challenged in our mouse model of inducible HSC depletion, which allows for a sustained reduction of HSC numbers in vivo (Schoedel et al., Blood 2016). We found that hematopoiesis continued normally in HSC-depleted mice for more than one year despite very low HSC numbers, and even exposure to acute hematopoietic stress was well tolerated by these animals. Using this animal model, we are currently investigating how the interplay between quiescent stem cells and proliferating progenitors as well as the hierarchical organization of hematopoiesis may counteract the acquisition and accumulation of somatic mutations in the hematopoietic system.
2. Mastocytosis and mast cell ontogeny
Mast cells are tissue resident immune cells of hematopoietic origin and are well established as effector cells of allergic reactions and anaphylaxis. Physiological functions of mast cells include host defense against helminth infections and insect venoms.
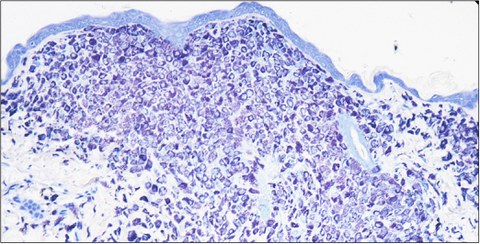
Mastocytosis is a rare human disease characterized by pathologic accumulations of mast cells in internal organs and skin. In pediatric mastocytosis, mast cell infiltrates are usually limited to the skin and this variant of the disease usually shows spontaneous and complete remission during adolescence. Adult patients suffering from systemic mastocytosis exhibit mast cell infiltrates in bone marrow and internal organs. In contrast to the pediatric variant, systemic mastocytosis does not show spontaneous remission in adult patients. Despite their heterogeneous clinical presentation and course, both disease entities share the same set of somatic mutations in the tyrosine kinase KIT (CD117), which result in constitutive activation of the receptor and sustained growth signals.
We have a long-standing interest in mast cells (Scholten et al., Transgenic Research 2008Weitzmann et al., Journal of Investigative Dermatology 2020) and the human disease mastocytosis. We have generated the KitD814V mouse model for mastocytosis (Gerbaulet et al. Blood 2011), which allows for conditional expression of the KitD814V mutation, the murine homolog of the somatic KITD816V mutation found in most human mastocytosis patients. By combining the KitD814Vflox allele with several cell type-specific or inducible Cre driver alleles, we can model different genetic mosaics of this somatic Kit mutation. For example, we can generate mice harboring the KitD814Vflox mutation in all blood lineages or, conversely, restrict mutant Kit to the mast cell lineage.
Similar to tissue-resident macrophages, mast cells are of dual ontogeny and are generated during two independent waves of either embryonic (“yolk sac”) or fetal ("definitive") hematopoiesis. The early wave of hematopoiesis originating from the yolk sac initially seeds peripheral tissues including skin with mast cells, but during late fetal and early postnatal development these cells are replaced by mast cells derived from definitive hematopoiesis. In an ongoing project, we are investigating how mast cell ontogeny modifies the effect of a somatic KIT mutation and may contribute to and explain the heterogeneous phenotype and course of mastocytosis.