ANTIMICROBIALS AND STRESS RESPONSE
Interdependence between different layers of the cell envelope stress response in Bacillus subtilis
Protection against antimicrobial peptides (AMPs) is key for bacterial survival in their natural habitat. While this defense often involves the parallel production of multiple, well-characterized resistance determinants, less is known about how these resistance modules interact and how they jointly protect the cell. To close this gap, we studied the interdependence between three known players of the envelope stress response of Bacillus subtilis when challenged with the lipid II cycle-inhibiting AMP bacitracin. The underlying regulatory network orchestrates the production of the ABC transporter BceAB, the UPP phosphatase BcrC and the phage-shock proteins LiaIH. Our analysis of the functional and regulatory interactions reveals a clear hierarchy, allowing us to discriminate between primary (BceAB) and secondary (BcrC and LiaIH) layers of bacitracin resistance. Strikingly, in a mutant devoid of the primary layer, the secondary layer is strongly induced and partially compensates for this mutation, thereby revealing the first direct role of LiaIH in bacitracin resistance. Conversely, deletions in the secondary layer provide novel insights into the feedback regulation of the Lia system, and underline a pivotal role of BcrC in maintaining cell wall homeostasis in the presence and absence of AMPs. Lastly, using flow cytometry, we show that the compensatory regulation between resistance layers can also explain how gene expression noise propagates amongst them. In accordance with previous studies, we confirmed that BceAB, BcrC and LiaIH actively react to the stimulus, because their promoter activity is stimulated by bacitracin, in contrast to constitutive promoters. We suggest that this active redundancy in the bacitracin resistance network of B. subtilis is a general principle to be found in many antibiotic resistance networks throughout the bacterial world. [Pubmed]
The LiaFSR system
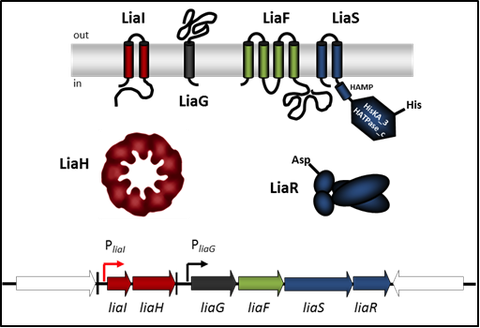
Figure 1: Schematic representation of the LiaFSR three-component system in B. subtilis. Genes and proteins are marked in dark blue/green (3CS), red (LiaRS target genes) and dark grey (unknown function). Genes flanking the lia operon are white. Promoter are represented with bent arrows, terminators by vertical bars.
The LiaFSR system is part of the CESR in B. subtilis. It consists of the TCS LiaSR and the LiaS-specific inhibitory membrane protein LiaF (Figure 1). The name LiaSR is derived from “lipid II cycle interfering antibiotic sensor and response regulator”. The LiaSR TCS is induced by cell wall antibiotics interfering with the lipid II cycle of cell wall biosynthesis (e.g. bacitracin, vancomycin, nisin) but also by detergents, organic solvents or more unspecific signals like secretion stress or filamentous phage infection. Under inducing conditions, the primary target of the activated response regulator LiaR is the liaI promoter controlling the liaIH operon (please see “LiaIH (phage shock protein-like) stress response” for more details).
In order to gain insight into the mechanism of stimulus perception by the histidine kinase LiaS, we surveyed sensor domain architecture and topology within the bacterial membrane, functional aspects related to this topology, and sequence and phylogenetic conservation of bacterial histidine kinases. Based on these criteria, we found that LiaS is a histidine kinase that detect stimuli via its membrane-spanning segments and – sometimes – an additional short extracellular loop. Based on the observation under non inducing conditions, the membrane protein LiaF keeps the HK LiaS strongly inactive, we assume that LiaF is potentially necessary for the detection and transfer of external stress signals via LiaS to the response regulator LiaR.
Methods
site-directed mutagenesis, bacterial two hybrid analysis, protein overexpression, membrane protein SPINE, co-IP, bioinformatics
People
Diana Wolf
Key references
(dann, wenn die Publikationsliste fertig ist, einfügen)
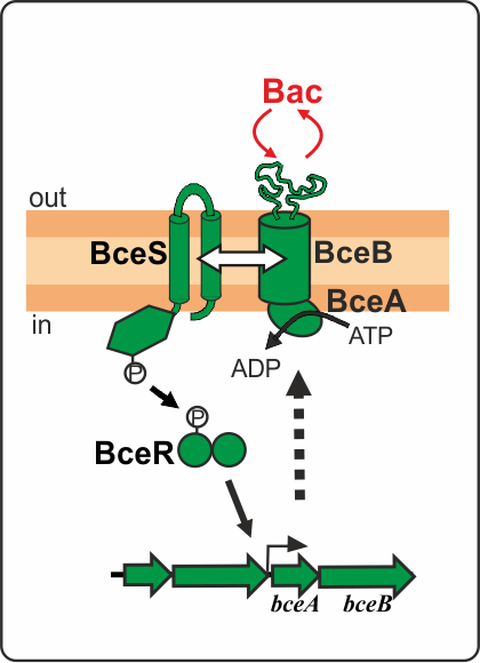
Figure 1. Schematic representation of the BceRSAB system from B. subtilis conferring bacitracin resistance.
BceRS-like TCSs are named after the paradigm systems of B. subtilis. These systems are genetically and functionally associated with BceAB-like ABC transporters consisting of an ATPase and permease subunits (Dintner et al. 2011, Gebhard and Mascher 2011). Together, BceRSAB-like modules are responsible for mediating high-level resistance against antimicrobial peptide (AMP) in Gram-positive bacteria. The resistance mechanism is as follows (Fig. 1): the ABC transporter BceAB detects the stimulus (bacitracin) and transfers the signal to the HK BceS, which does not function as a direct sensor but rather as a signal transfer relay to BceR. Activation of the RR BceR then induces the expression of bceAB and thus ensures antibiotic resistance. Once the inducing AMP is eliminated, the system is switched off. BceRSAB-like systems therefore represent highly efficient AMP-specific detoxification modules.
Maintaining wiring specificity in TCS signaling is critical for survival but is often challenged by the high degree of similarity at the sequence and structural levels, since new TCS pathways usually arise by gene duplication events (Capra and Laub 2012). Because of the high degree of conservation of the transmitter/receiver pairs, spurious crosstalk could be expected but in nature rarely occurs since pathways are sufficiently insulated from each other. The insulation and specificity are based primarily on molecular recognition. To ensure specific phosphotransfer between newly duplicated HK and RR pairs – and thereby avoiding crosstalk between the new pathways - interacting amino acid pairs between cognate HK and RR pairs need to be exchanged during the diversification process. By this intermolecular coevolution, insulation between the new pathways is gradually increased while simultaneously ensuring the functionality of signal transfer within each pathway.
B. subtilis possesses three paralogous Bce-like TCSs, which seem to be derived from gene duplication events. In the course of evolution, they have evolved to different pathways, responding to and mediating resistance against different AMPs: BceRS (bacitracin), PsdRS (nisin) and YxdJK (LL-37) (Staroń et al. 2011). The probiotic bacterium L. casei BL23 possesses two paralogous BceRS-like TCS, TCS09 and TCS12 (Revilla-Guarinos et al. 2013).
Both TCSs are located next to genes encoding ABC transporters (ABC09 and ABC12). A third BceAB-like ABC transporter not genetically associated with a TCS (orphan ABC) is also encoded in the genome of L. casei BL23.
We have different ongoing research projects focused in these Bce systems:
- Further characterization of the regulatory network of Bce systems from L. casei.
- Studies with yxdJKLM from B. subtilis and its role in antimicrobial peptide stress response.
- Identification of the molecular specificity determinants that allow insulation of the highly similar Bce-like TCS pathways at the HK-RR and RR-promoter interface.
For these projects we perform a double in vivo/in vitro approach. We implement B. subtilis as an heterologous expression system for L. casei. Previous results from the group proved B.subtilis to be a good heterologous expression system for Enteroccocus faecalis (Fang et al. 2014).
People
Diana Wolf
Key references
Fritz, G., S. Dintner, N.S. Treichel, J. Radeck, U. Gerland, T. Mascher, and S. Gebhard, A New Way of Sensing: Need-Based Activation of Antibiotic Resistance by a Flux-Sensing Mechanism. MBio, 2015. 6(4): p. e00975. [Pubmed]
Revilla-Guarinos, A., S. Gebhard, T. Mascher, and M. Zúñiga, Defence against antimicrobial peptides: different strategies in Firmicutes. Environ Microbiol, 2014. 16(5): p. 1225-37. [Pubmed]
Fang, C., E. Stiegeler, G.M. Cook, T. Mascher, and S. Gebhard, Bacillus subtilis as a platform for molecular characterisation of regulatory mechanisms of Enterococcus faecalis resistance against cell wall antibiotics. PLoS ONE, 2014. 9(3): p. e93169. [Pubmed]
Mascher, T., Bacterial (intramembrane-sensing) histidine kinases: signal transfer rather than stimulus perception. Trends Microbiol, 2014. 22(10): p. 559-65. [Pubmed]
Revilla-Guarinos, A., S. Gebhard, C. Alcántara, A. Staroń, T. Mascher, and M. Zúñiga, Characterization of a Regulatory Network of Peptide Antibiotic Detoxification Modules in Lactobacillus casei BL23, in Appl. Environ. Microbiol.2013. [Pubmed]
Schrecke K., Staroń A., and M. T., Two-component Signaling in the Gram-positive Envelope Stress Response: Intramembrane-sensing Histidine Kinases and Accessory Membrane Proteins, in Two-component Systems in Bacteria. Book., G. Roy Gross and Dagmar Beier University of Würzburg, Editor 2012, Caister Academic Press. Two-component Systemes in Bacteria|Book. EAN: 9781908230089. [Publisher]
Dintner, S., A. Staroń, E. Berchtold, T. Petri, T. Mascher, and S. Gebhard, Coevolution of ABC transporters and two-component regulatory systems as resistance modules against antimicrobial peptides in Firmicutes Bacteria. J. Bacteriol., 2011. 193(15): p. 3851-62. [Pubmed]
Staroń, A., D.E. Finkeisen, and T. Mascher, Peptide antibiotic sensing and detoxification modules of Bacillus subtilis. Antimicrob. Agents Chemother., 2011. 55(2): p. 515-25. [Pubmed]
Gebhard, S. and T. Mascher, Antimicrobial peptide sensing and detoxification modules: unravelling the regulatory circuitry of Staphylococcus aureus. Mol. Microbiol., 2011. 81(3): p. 581-7. [Pubmed]
Rietkötter, E., D. Hoyer, and T. Mascher, Bacitracin sensing in Bacillus subtilis. Mol. Microbiol., 2008. 68(3): p. 768-85. [Pubmed]
Jordan, S., M.I. Hutchings, and T. Mascher, Cell envelope stress response in Gram-positive bacteria. FEMS Microbiology Reviews, 2008. 32(1): p. 107-146. [Pubmed]
Mascher, T., N.G. Margulis, T. Wang, R.W. Ye, and J.D. Helmann, Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol., 2003. 50(5): p. 1591-604. [Pubmed]
