Enzymatic fixation of carbon dioxide
Carbon dioxide is known as a “bad guy”, most prominently for causing global warming and climate change. On the other hand, it can act as a carbon source or electron acceptor if you think about photosynthesis or chemolithotrophic growth of microorganisms. It can also act as a resource to synthetize chemical building blocks - there are a few approaches for the fixation of carbon dioxide in industry by chemical procedures, e.g. synthesis of urea or salicylic acid. General main disadvantages of these processes are the use of very expensive catalysts like Rhodium or Iridium and requirement for high pressures and temperatures. A biocatalytic approach, however, can take place under normal atmospheric pressure and room temperature. Hence, it would be a green alternative to chemical processes and could also save money.
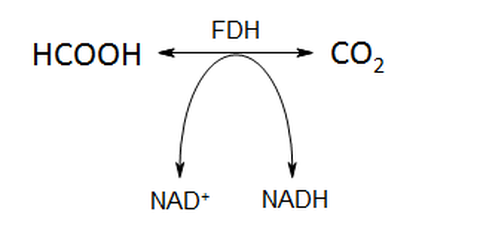
Fig. 1: Schematic reaction of FDH with cofactor
Formate dehydrogenases (FDHs) belong to the enzyme group of oxidoreductases and catalyze the oxidation of formate to carbon dioxide (Fig.1). In this reaction, electrons are released which are used to reduce NAD+ (NAD-dependent FDHs). This makes FDHs suitable for cofactor regeneration systems. On the other hand, the reverse reaction (fixation of carbon dioxide to formate) shows only very little activities. The aim of the project is to identify enzyme variants, which show higher activity in the reduction of carbon dioxide and could therefore be of use for an efficient system to transform a greenhouse gas into valuable chemical compounds.
Bullet points:
- Investigation of CO2 reducing activity of FDH variants from described microorganism
- Isolation and identification of new FDH variants (enrichment culture)
- Mutagenesis of enzyme candidates to enhance reductive activity
- Studies with alternative cofactors
- Enzyme complex design - use of other electron donors with a suitable redox potential
