Research projects
The general interest of our group is to understand the mechanisms by which cells collectively organize to form complex patterns and morphologies in developing tissues. How does collective behavior emerge in cell assemblies? How do mechanical processes like cell adhesion or force generation influence tissue organization? And how are these mechanical processes linked to the chemical signals that orchestrate tissue development? We address these questions by combining genetics with live imaging, quantitative image analysis and biophysical approaches using the fruit fly Drosophila melanogaster as a model organism. Moreover, in collaborations with physicist and mathematicians, we use our quantitative data to build computational models of tissue organization.
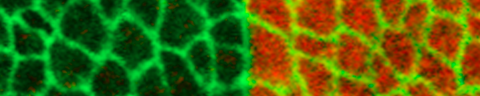
Physical mechanisms shaping compartment boundaries

Compartment boundaries in Drosophila
Epithelial sheets are excellent model systems to understand how the collective behavior of cells results in complex patterns and morphologies. One example of the collective behavior of cells within epithelia is the formation of straight and sharp tissue interfaces (termed compartment boundaries) that separate groups of cells with distinct identities and fates during animal development. The formation of compartment boundaries is important because signaling between neighboring compartments elicits the formation of organizing centers along the boundaries that instruct growth and patterning of the tissue. We have previously shown that the sorting out of cells at the boundary between anterior and posterior compartments of the developing Drosophila wing is controlled by a transcriptional response to the Hedgehog and BMP signaling pathways. Recent physical approaches, including the ablation of bonds between cells using UV laser light, have revealed that mechanical tension on cell bonds is elevated along the anterior-posterior and dorso-ventral compartment boundaries of the developing wing. The local increases in cell bond tension is induced by local signaling across the compartment boundary. Live imaging combined with quantitative image analysis then showed that the local increase in cell bond tension biases cell rearrangements during cell intercalations to maintain the characteristic straight shape of the compartment boundary. Computer simulations performed in collaboration with the group of Frank Jülicher at the Max Planck Institute for the Physics of Complex Systems show that a local increase of cell bond tension suffices to maintain straight interfaces between compartments. Our work suggests that the large-scale shape of compartment boundaries emerges from the collective behavior of many cells that locally exchange biochemical signals and regulate mechanical tension.
Control of epithelial cell shape by BMP signalling
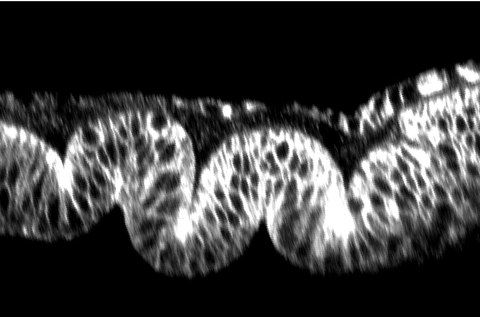
Hinge fold formation in the Drosophila wing disc
Epithelial shape is an emergent property of the constituent cells. Collective changes in the shape of cells lead at larger scale to changes in tissue morphology. These cell shape changes arise from local changes in cell mechanical properties, including cell adhesion and contractility of the actomyosin cytoskeleton. We have previously shown that the BMP /Dpp signal transduction pathway, once thought to promote cell survival, instead controls cytoskeletal organization, cell shape, and integrity of the developing wing epithelium in Drosophila. Our more recent results demonstrate that the BMP /Dpp signal transduction pathway controls cell shape and epithelial architecture through controlling the activity and subcellular localization of the small GTPase Rho1 and the motor protein non-muscle myosin II. These results provide a link between chemical signaling and cell mechanics in shaping epithelial sheets.
Emergence of planar cell polarity
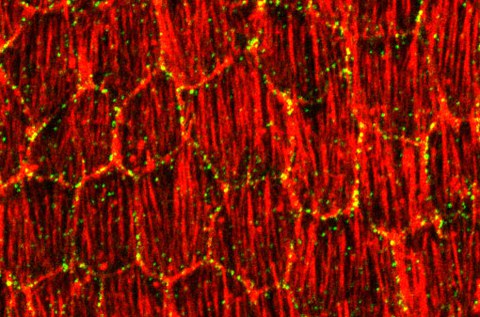
Planar polarity of actin filaments in the follicle epithelium
Polarizing cells in the plane of tissues is common in animals. In the Drosophila ovary, this planar polarity is evident by the stereotypic orientation of actin filaments in follicle cells. We have shown that this stereotypic orientation of actin filaments depends on the atypical cadherin Fat2. Fat2 protein distribution is itself polarized within follicle cells, providing a mechanism how planar polarity is established. Moreover, in collaboration with Len Pismen at the Technion in Haifa (Israel), we have based on our quantitative experimental data devised a computational model to explain how planar polarity can emerge within epithelia.
The planar organization of actin filaments and extracellular matrix fibers constitutes a 'molecular corset' that contributes to the elongation of the follicles (egg chambers) inside the ovary. The planar organization of extracellular matrix fibers had previously been proposed to dependent on the whole tissue rotation of the follicle. However, our recent results show that the planar organization of extracellular matrix fibers and egg chamber elongation can be genetically uncoupled from egg chamber rotation.
Selected publications:
-
Michel, M. and Dahmann, C. (2016) Memorizing shape to orient cell division. Developmental Cell 36, 589-590 [Pubmed]
- Aurich, F. and Dahmann, C. (2016) A mutation in fat2 uncouples tissue elongation from global tissue rotation. Cell Reports 14, 2503-2510(11) [Pubmed]
-
Rudolf, K., Umetsu, D., Aliee, M., Sui, L., Jülicher, F., and Dahmann, C. (2015) A local difference in Hedgehog signal transduction increases mechanical cell bond tension and biases cell intercalations along the Drosophila anteroposterior compartment boundary. Development 142, 3845-58. [Pubmed]
-
Umetsu, D., Aigouy, B., Aliee, M., Sui, L., Eaton, S., Jülicher, F., and Dahmann, C. (2014) Local increases in mechanical tension shape compartment boundaries by biasing cell intercalations. Current Biology, Vol. 24, No15 (2014) [Pubmed]
-
Umetsu, D., Dunst, S. and Dahmann C. (2014) An RNA Interference Screen for Genes Required to Shape the Anteroposterior Compartment Boundary in Drosophila Identifies the Eph Receptor. PLOS One, 9(12) (2014) [Pubmed]
-
Viktorinova, I. and Dahmann, C. (2013) Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Current Biology, 23(15) (2013) [Pubmed]
-
Aliee, M., Röper, J.-C., Landsberg, K. P., Pentzold, C., Widmann, T. J., Jülicher, F., and Dahmann, C. (2012) Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Current Biology, 22, 967-976.
-
Dahmann, C., Oates, A. C., and Brand, M. (2011) Boundary formation and maintenance in tissue development. Nature Reviews Genetics, 12, 43-55.
-
Viktorinova, I., Aigouy, B., Pismen, L. M., and Dahmann, C. (2011). Modelling planar polarity of epithelia: the role of signal relay in collective cell polarization. J. R. Soc. Interface, 8, 1059-63.
-
Landsberg, K. P., Farhadifar, R., Ranft, J., Umetsu, D., Widmann, T. J., Bittig, T., Said, A., Jülicher, F., and Dahmann, C. (2009). Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Current Biology, 19, 1950-1955.
-
Viktorinova, I., König, T., Schlichting, K., and Dahmann, C. (2009). The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development, 136, 4123-4132.
-
Widmann, T. J. and Dahmann, C. (2009). Dpp signaling promotes the cuboidal to columnar cell shape transition of Drosophila wing disc epithelia through regulating Rho1. Journal of Cell Science, 122, 1362-1373.
-
Dahmann, C. (Editor) (2008). Drosophila: Methods and Protocols, Humana Press, Totowa.
-
Shen, J. and Dahmann, C. (2005). Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science, 307, 1789-1790.