Dec 13, 2021
Boosting the Electrocatalytic Conversion of Nitrogen to Ammonia on Metal-Phthalocyanine-Based Two-Dimensional Conjugated Covalent Organic Frameworks
Electrochemical nitrogen reduction reaction (NRR), particularly in conjunction with renewable energy, is regarded as a promising and sustainable alternative to the current high temperature/pressure Harber-Bosch process for the production of ammonia under ambient conditions. The current electrochemical NRR process suffers from extremely sluggish kinetics and low selectivity due to the high energy barriers of N2 activation and the faster kinetics of the competing hydrogen evolution reaction (HER) in the similar potential ranges, respectively. Thus, extensive efforts have been devoted to explore highly active and selective electrocatalysts for boosting NRR. Thereinto, heterogeneous metal-heteroatom-doped carbon-rich electrocatalyst represents one attractive alternative because of its high durability and recyclability, as well as its ease of integration into the electrode. Nevertheless, the inhomogeneity and ambiguity of the catalytic structure of metal-nitrogen-doped carbon posed major challenges in synergistically improving their activity and selectivity toward high NRR efficiency. Moreover, their inherently less defined catalytic environment hinders the fundamental studies on the reaction mechanism.
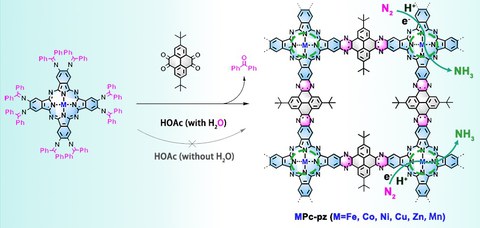
In order to address these challenges, researchers from Technical University of Dresden (Chair for Molecular Functional Materials) and collaborators have presented the first case of two-dimensional conjugated covalent organic frameworks (2D c-COFs) incorporated with M-N4-C centers as sufficient catalysts, achieving simultaneously enhanced activity and selectivity of electrocatalytic NRR to ammonia. Such 2D c-COFs are synthesized based on metal-phthalocyanine (M = Fe, Co, Ni, Mn, Zn and Cu) and pyrene units bonded by pyrazine linkages. The 2D c-COFs with Fe-N4-C center exhibit higher ammonia yield rate (33.6 mg h-1mg-1cat) and Faradaic efficiency (FE, 31.9 %) at -0.1 V vs. reversible hydrogen electrode than those with other M-N4-C centers, making them among the best NRR electrocatalysts (yield rate >30 mg h-1mg-1cat and FE >30 %). In-situ X-ray absorption spectroscopy, Raman spectroelectrochemistry and theoretical calculations unveil that Fe-N4-C centers act as catalytic sites. They show unique electronic structure with localized electronic states at Fermi level, allowing for the stronger interaction with N2, thus faster N2 activation and NRR kinetics than other M-N4-C centers. Our work opens the possibility of developing metal-nitrogen-doped carbon-rich 2D c-COFs as superior NRR electrocatalyst and provides an atomic understanding of NRR process on M-Nx-C based electrocatalysts for designing high-performance NRR catalysts
Reference: Haixia Zhong, Mingchao Wang, Mahdi Ghorbani-Asl, Jichao Zhang, Khoa Hoang Ly, Zhongquan Liao, Guangbo Chen, Yidan Wei, Bishnu P. Biswal, Ehrenfried Zschech, Inez M. Weidinger, Arkady V. Krasheninnikov, Renhao Dong,and Xinliang Feng. Boosting the Electrocatalytic Conversion of Nitrogen to Ammonia on Metal-Phthalocyanine-Based Two-Dimensional Conjugated Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 47, 19992–20000.
This work is financially supported by EU Graphene Flagship (GrapheneCore3, No. 881603), ERC starting grant (FC2DMOF, No. 852909), ERC Consolidator Grant (T2DCP), Coordination Networks: Building Blocks for Functional Systems (SPP 1928, COORNETs), and CRC 1415 (Chemistry of Synthetic Two-Dimensional Materials, No. 417590517), as well as the German Science Council and Center for Advancing Electronics Dresden (cfaed). R.D. thanks Taishan Scholars Program of Shandong Province (tsqn201909047). H.Z. gratefully acknowledges funding from the Alexander von Humboldt Foundation. I.M.W. acknowledges the Cluster of Excellence UniSysCat (EXC 2008/1-390540038). We acknowledge Dresden Center for Nanoanalysis (DCN) at TUD and Dr. Petr Formanek (Leibniz Institute for Polymer Research, IPF, Dresden) for the use of facilities. We thank the scientists at beamline BL14W1 and BL15U1 of the Shanghai Synchrotron Radiation Facility for the XAFS measurements. We appreciate Prof. Thomas Heine and Hung-Hsuan Lin for providing the structural model. We also appreciate Na Zhou for IC measurement from Changchun institute of applied chemistry (CIAC). We thank Prof. Xiaodong Zhuang for the in-situ XAS electrochemical cell setup. The computational support from the HZDR computing cluster, Technical University of Dresden cluster (TAURUS), High Performance Computing Center (HLRS) in Stuttgart, Germany, and CSC Finland, is gratefully appreciated.