11.08.2020
On-Surface Synthesis of Non-Benzenoid Nanographenes by Oxidative Ring-Closure and Ring-Rearrangement Reactions
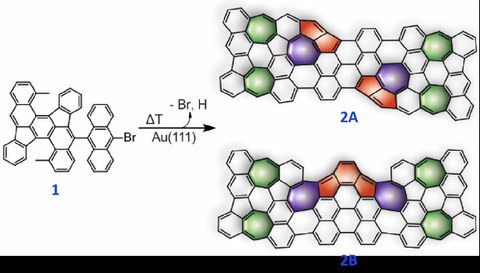
Figure 1. Synthesis of Non-Benzenoid nanographenes 2A and 2B. Colored rings highlight the formation of heptagons via oxidative ring closure (depicted in green) and cyclodehydrogenation (depicted in blue) and pentalene (2A) and as-indacene (2B) moieties via ring rearrangement (depicted in red).
Nanographenes (NGs) have gained increasing attention due to their immense potential as tailor-made organic materials for nanoelectronics and spintronics. They exhibit a rich spectrum of physicochemical properties that can be tuned by controlling the size or the edge structure or by introducing structural defects in the honeycomb lattice. Moreover, NGs containing unpaired electrons have received increased interest in recent years due to their potential applications in organic spintronics. To date, most reported NGs consist exclusively of benzenoid rings. Nevertheless, the controlled introduction of structural defects, i.e. oddmembered polycycles (pentagons or heptagons), in the honeycomb lattice may have a considerable effect on the physicochemical properties of NGs, arising from local changes in strain and conjugation.
Recently, researchers from Technische Universität Dresden (Chair of Molecular Functional Materials) and collaborators (EMPA, Zurich, and HKU, Hong Kong) reported the design and on-surface synthesis of NGs containing several odd-membered polycycles induced by a thermal procedure on Au(111). Two nonbenzenoid NGs (2A and 2B, Figure 1) containing four embedded azulene units in the polycyclic framework were achieved via onsurface oxidative ring-closure reactions. Interestingly, surface-catalyzed skeletal ring rearrangement reactions in NGs 2A and 2B were observed, which lead to the formation of additional heptagonal rings as well as pentalene and as-indacene units, respectively. The chemical structures of 2A and 2B have been elucidated by STM and nc-AFM. STS studies reveal that 2A and 2B exhibit narrow experimental frontier electronic gaps of 0.96 and 0.85 eV on Au(111), respectively, with moderate open-shell characters. These results reported herein motivate the rational synthesis of larger non-benzenoid ring topologies as functional centers toward the perspective of engineering graphene-based devices.
Reference:
Thorsten G. Lohr, Jose I. Urgel,* Kristjan Eimre, Junzhi Liu,* Marco Di Giovannantonio, Shantanu Mishra, Reinhard Berger, Pascal Ruffiffiffieux, Carlo A. Pignedoli, Roman Fasel,* and Xinliang Feng.* J. Am. Chem. Soc. 2020, 142, 13565-13572.
This work was supported by the Swiss National Science Foundation (200020_182015), the European Union’s Horizon 2020 research and innovation programme (GrapheneCore2 785219), the Office of Naval Research (N00014-18-1-2708), and the Swiss National Centre for Computational Design and Discovery of Novel Materials (MARVEL). The Swiss National Supercomputing Centre (CSCS) under project ID s746 and s904 is acknowledged for computational resources. The DFGNSFC Joint Sino-German Research Project (EnhanceNano), Center for Advancing Electronics Dresden (cfaed), the European Social Fund, and the Federal State of Saxony (ESF-Project GRAPHD, TU Dresden) are acknowledged for financial support. J.L, is grateful for the startup funding from The University of Hong Kong and the funding support from ITC to the SKL. T.G.L. gratefully acknowledges the International Excellence Graduate School on Emerging Materials and Processes Korea (iEGSEMP Korea) in the context of TU Dresden’s institutional strategy The Synergetic University.
