Aug 21, 2020
Seeing is believing: How do grain boundaries look like in 2D Polymers?
Since the dawn of Materials Science, researchers in this field have been fascinated by the beauty of crystals, in which all atoms appear at the right position in the right order. But, even the glowing diamonds on the wedding rings, the perfect symbol of eternal love and commitment, are never as perfect as we thought. Crystals, regardless of natural or synthetic, contain defects, where the atoms are not arranged as they are supposed to be or grain boundaries as the borderline between two crystalline domains. The study on the imperfections has been a vital topic in nanomaterials and nanotechnology because even a trivial change in the local structure may bring about substantial variation in materials physical and chemical properties. Since the imperfections can be small as missing tiny atoms are, to see how defects look like, and to establish the connection between defects and the resulting change in the properties belongs to the most challenging tasks ever met by materials scientists. With beginning of this century, just after the revolution in resolution due to the realization of “glasses” for electron microscope’s main lenses, transmission electron microscopy (TEM) became a powerful tool to guide into the world of single atoms. Such modern microscopes can now resolve structural features down to the sub-Angstrom scale (1 Angstrom = 10-10 meter = the order of the size of atoms), and therefore TEM developed now to a close and reliable companion to unravel also two-dimensional (2D) materials starting with graphene, the one carbon atom thin wonder material discovered in 2004 and awarded with the Nobel prize in 2010.
Recent years have witnessed the rise of a new star on the vast sky of synthetic 2D materials, namely, 2D polymer. Contrast to traditional linear polymers discovered 100 years ago by Staudinger (Chem. Ber. 1920, 53, 1073), 2D polymers consist of repeating building blocks in a 2D plane linked by strong covalent bonds, forming a sheet-like network. The enormous chemical and structural diversity of the building blocks (i.e., monomers) and linkage types provide the scientists with a playground filled with infinite creative possibilities. That means the synthetic polymer materials can be pre-designed and 'programmed' for desirable applications.
But, like every other crystalline material, 2D polymers also contain imperfections. Strikingly, how the imperfections really look like remains a mystery till now, even with the best TEMs available. Because TEM uses a high-energy electron beam as the imaging source, whether or not we can image the structure depends not only on the attainable resolution of the microscope. It largely depends also on the material under study itself. Can it remain intact during the imaging process? 2D polymers, unfortunately, like many other organic materials, quickly 'burn to ashes' when the electron beam is switched on. How can we image 2D polymers then? Well, like if you want to read a book in the middle of the night, instead of turning on a surgical light with high brightness, a small candle is probably enough. When translated, we only need to provide just a sufficient amount of electrons to obtain the structural information before the material is destroyed. A simple idea but not easy to realize! A group of scientists from Universität Ulm and Technische Universität Dresden have successfully observed grain boundary structures in 2D polyimine with an unprecedented resolution of 2.3 Angstrom (see Figures 1 and 2). The 2D polyimine was prepared by our recently-developed surfactant-monolayer-assistant interfacial synthesis (SMAIS), which took the synthesis of highly crystalline 2D polymers to a new level. Strikingly, based on the known-knowledge from inorganic 2D materials, such as graphene and transition metal dichalcogenides, high-energy point defects should appear at the grain boundaries between two crystalline domains. However, as demonstrated using our computer simulations, for 2D polymers, the two crystalline domains tend to be connected via flexible covalent bonds at the grain boundaries, suggesting that the local properties of 2D polymers are more robust compared to traditional materials: while in the latter a lot of binding energy is lost due to the imperfection of the bonding between the atoms across the grain boundaries, the structural flexibility of the molecules in the 2D polymer strongly reduces this energy penalty and thus limits the impact of the grain boundary on the stability, but also on the chemical and physical properties of the 2D polymer.
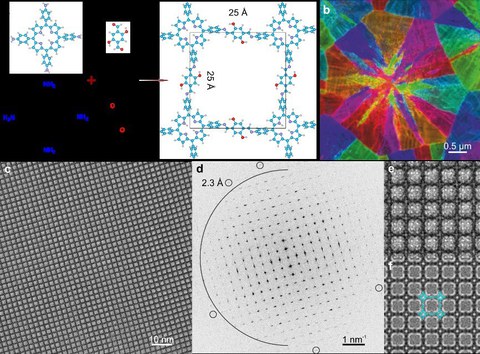
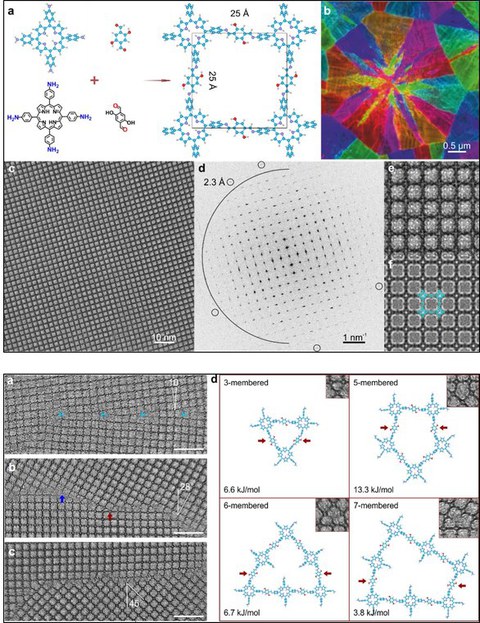
Abbildung 1. (a) Atommodell von 2D Polyimin; (b) Kornorientierungskarte. (c) Unbearbeitetes AC-HRTEM-Bild, aufgenommen mit einer Elektronendosis von 100 e-/Å2. (d) Fast-Fourier-Transformationsmuster von (c). Der Halbkreis markiert die Ortsfrequenz von 4 nm-1 (d.h. 2,5 Å). Die kleinen Kreise markieren die Reflexionen bei der Nyquist-Frequenz 4,35 nm-1 (d.h. 2,3 Å). (e) Vergrößerte Abbildung von (a). (f) Simuliertes Bild mit dem überlagerten Atommodell.
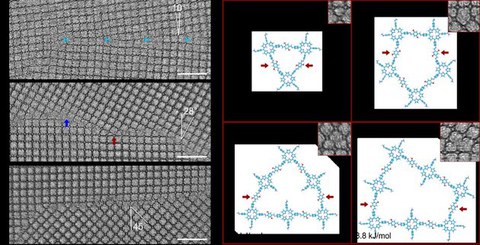
Figure 2. (a) Low-angle grain boundary with a misorientation of 10°. (b) High-angle grain boundary with a misorientation of 28°. The red and blue arrows mark TAPP and DhTPA terminations, respectively. (c) High-angle grain boundary with a misorientation of 45°. (d) DFTB models of n-membered rings formed by boundary reconstruction, and the relative formation energy per inter-grain DhTPA. The red arrows mark the inter-grain DhTPAs. Scale bar: 10 nm.
These results are published on August, 14th, 2020 in Science Advances. It makes a fabulous start of the Collaborative Research Centre 1415 "Chemistry of Synthetic Two-Dimensonal Materials".
Reference
H. Qi, H. Sahabudeen, B. Liang, M. Položij, M. Addicoat, T. Gorelik, M. Hambsch, M. Mundszinger, S. Park, B. Lotsch, S. Mannsfeld, Z. Zheng, R. Dong, T. Heine, X. Feng, Ute Kaiser, “Near-atomic-scale observation of grain boundaries in a layer-stacked two-dimensional polymer", Sci. Adv. 2020.
H. Sahabudeen and B. Liang contributed equally to this work. H. Qi, T. Heine, X. Feng and U. Kaiser are the corresponding authors of this joint publication.