Mar 30, 2020
The unprecedented power of the reductant: versatile modulation of noble metal aerogels for various electrocatalysis processes
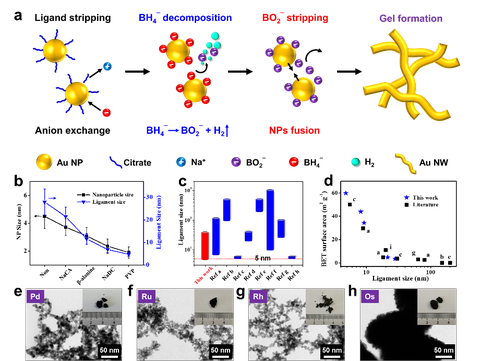
(a) The underlying mechanisms of excessive-NaBH4-directed gelation method. (b) Modulation of the ligament size by ligand chemistry, and (c-d) comparison of ligament size and specific surface areas of gold aerogels from this work and literature.
TU Dresden chemists create noble metal aerogels with eight metals for various electrocatalysis processes. Their work has now been published in the renowned journal Nature Communications.
As a newcomer in aerogel family, noble metal aerogels (NMAs) feature attributes of both, noble metals and aerogels, thus manifesting advantages in various fields, especially for energy and electrocatalysis. However, the composition of NMAs is still largely constrained and the structure control is far from satisfactory. This is largely due to the insufficient understanding of the gelation mechanisms and the involved key ingredients, such as reductants and ligands.
Humboldt research fellow Dr. Ran Du is a postdoc in the physical chemistry group of Prof. Alexander Eychmüller at TU Dresden since 2017. In collaboration with Dr. Jinying Wang from Purdue University, USA, and Dr. Yue Hu from Wenzhou University, China, Dr. Du found multiple roles of reductants in the process of preparing NMAs. By unveiling multiple roles of reductants and deeply investigating the underlying formation mechanisms, the international team developed an extremely powerful excessive-reductant-directed gelation strategy, allowing the fabrication of gold aerogels with a record-high surface area and the expansion of available elements to eight metals. In this light, high-performance electrocatalysts for the oxygen evolution reaction (OER) as used for water splitting, and the ethanol oxidation reaction (EOR) as used for fuel cells are acquired.
Previously the same group reported the salt-directed gelation and detailed the mechanisms behind (Sci. Adv. 2019). Reductants for creating noble metal nanoparticles (NPs), for example sodium borohydride (NaBH4), are also salts, hence, the expectation of Ran Du and his team was that this salt should also be able to trigger the gelation. By experimental and theoretical approaches, they revealed multiple functions of broad electrolyte-type reductants, i.e., as reducing agents, ligands, and salting-out agents. Moreover, they demonstrated the uniqueness of NaBH4 , which can greatly promote the gelation process.
With a deep understanding of the underlying mechanism, a powerful excessive-reductant-directed gelation strategy was accordingly developed, unlocking versatile manipulations of NMAs. By activating ligand chemistry, gold aerogels with a record-high specific surface area — as high as 59.8 m2 g-1 — were acquired. The composition space of NMAs was expanded to all common noble metals (gold (Au), silver (Ag), palladium (Pd), platinum (Pt), ruthenium (Ru), rhodium (Rh), iridium (Ir), and osmium (Os)). Unexpected spontaneous combustion phenomena were observed for Ru, Rh, and Au-Ru aerogels.
The wide available NMAs facilitate optimization of various important electrocatalysis processes. Ran Du and his team observed an unconventional ligand-enhancing effect boosting the electrocatalytic ethanol oxidation reaction (EOR) performance of the gold-palladium (Au-Pd) aerogel by 50% and displaying a forward current density of up to 7.7 times higher than that of commercial palladium on carbon (Pd/C). Additionally, gold-iridium (Au-Ir) core-shell-structured aerogels were characterized as excellent OER electrocatalysts outperforming commercial Ir/C and IrO2 catalysts in both alkaline and acid environment.
“We believe the current work not only strides a big step towards insightful understanding the gelation mechanisms, manipulating the microstructures, and enriching the composition library of NMAs, but also opens a new dimension for devising high-performance electrocatalysts by taking advantage of the ligand effects,” assumes chemist Ran Du.
Original Publication:
Du, R., Wang, J., Wang, Y., Hübner, R., Fan, X., Senkovska, I., Hu, Y., Kaskel, S., Eychmüller, A., (2020). Unveiling Reductant Chemistry in Fabricating Noble Metal Aerogels for Superior Oxygen Evolution and Ethanol Oxidation. Nat. Commun., DOI: 10.1038/s41467-020-15391-w.
Media inquiries to:
Nicole Gierig
Public Relations Advisor
School of Science
TU Dresden
Email: