May 31, 2023
New Publication out !
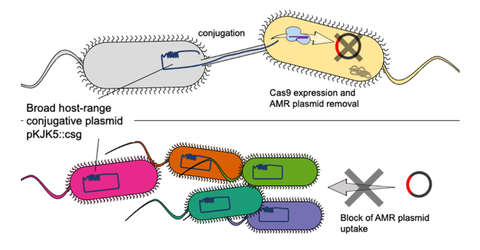
A broad host-range CRISPR- Cas9 delivery tool was developed and used to conjugatively remove antimicrobial resistance (AMR) plasmids (top) and protect a series of coliform and Pseudomonas isolates from AMR plasmid uptake (bottom).
A new tool which could help reduce the spread of antimicrobial resistance is showing early promise, through exploiting a bacterial immune system as a gene editing tool.
Antimicrobial resistance is a major global threat, with nearly five million deaths annually resulting from antibiotics failing to treat infection, according to the World Health Organisation.
Bacteria often develop resistance when resistant genes are transported between hosts. One way that this occurs is via plasmids – circular strands of DNA, which can spread easily between bacteria, and swiftly replicate. This can occur in our bodies, and in environmental settings, such as waterways.
The Exeter team harnessed the CRISPR-Cas gene editing system, which can target specific sequences of DNA, and cuts through them when they are encountered. The researchers engineered a plasmid which can specifically target the resistance gene for Gentamicin – a commonly used antibiotic.
In laboratory experiments, the new research, published in Microbiology, found that the plasmid protected its host cell from developing resistance. Furthermore, researchers found that the plasmid effectively targeted antimicrobial resistant genes in hosts to which it transferred, reversing their resistance.
Lead author David Walker-Sünderhauf, of the University of Exeter, said: “Antimicrobial resistance threatens to outstrip covid in terms of the number of global deaths. We urgently need new ways to stop resistance spreading between hosts. Our technology is showing early promise to eliminate resistance in a wide range of different bacteria. Our next step is to conduct experiments in more complex microbial communities. We hope one day it could be a way to reduce the spread of antimicrobial resistance in environments such as sewage treatment plants, which we know are breeding grounds for resistance.”
The research is supported by GW4, the Medical Research Council, the Lister Institute, and JPI-AMR. The paper is entitled ‘Removal of AMR plasmids using a mobile, broad host-range, CRISPR-Cas9 delivery tool’, and is published in Microbiology. (Author Louise Vennells)
