PQQpat - Establishing a pyrroloquinoline quinone-dependent pathway for efficient alcohol metabolism in Escherichia coli
Ethylene glycol (EG) and methanol are promising liquid feedstocks for industrial bioproduction. Despite the efforts to enable the workhorse Escherichia coli to metabolize EG or methanol, engineered strains grow at rates significantly lower than for other species naturally capable of assimilating these alcohols. In aerobic EG- and methanol pathways, the alcohol is first oxidized into the corresponding aldehyde (Figure 1). In engineered E. coli, this step is carried by NAD-dependent alcohol dehydrogenase enzymes. But because such reactions are thermodynamically unfavorable, processing of the respective aldehyde (mainly through synthetic metabolic pathways) becomes very difficult due to their extremely low concentrations.
In nature, this thermodynamic barrier is overcome by using NAD-DH dependent alcohol assimilation at high temperatures. At mesophilic temperatures, natural alcohol-assimilation microorganisms employ alcohol oxidase (AOX) or pyrroloquinoline quinone (PQQ)-dependent alcohol dehydrogenases (PQQ-DH) which irreversibly oxidize the alcohol. But since AOX-dependent alcohol oxidation only produces heat but no redox co-factor which could be used to drive biosynthetic reactions or to produce ATP, PQQ-DH dependent systems are a preferable choice from a metabolic engineering point of view.
In this project, we first aim to elucidate the molecular components and mechanisms implicated in PQQ-based alcohol oxidation systems. On the basis of this knowledge, challenges of EG / methanol assimilation will be addressed by installing in E. coli a non-canonical pyrroloquinoline quinone (PQQ)-dependent system which can irreversibly oxidize the alcohol(s).
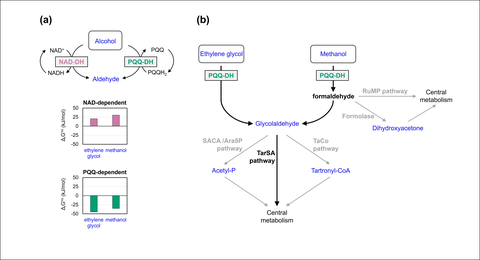
Fig. Bacterial metabolic pathways for alcohol assimilation proceed through dehydrogenases.
(a) The NAD-dependent oxidation of methanol or ethylene glycol to corresponding aldehydes is thermodynamically unfavorable. In fermentation processes, this requires the utilization of high concentrations of the respective alcohol rendering complete utilization of the substrate nearly impossible. An attractive solution to this challenge would be the utilization of pyrroloquinoline quinone-dependent alcohol dehydrogenases (PQQ-DH) which possess favorable thermodynamics.
(b) The implementation of PQQ-dependent alcohol dehydrogenase for ethylene glycol or methanol assimilation is compatible with any state-of-the-art natural/canonical or non-natural pathway. Natural pathways: TarSA (tartronic semialdehyde pathway), RuMP (ribulose monophosphate pathway). Synthetic pathways: SACA (synthetic acetyl-CoA pathway), Ara5P (D-arabinose-5P pathway), TaCo (tartronyl-CoA pathway), formolase pathway.
Project management:
 © Mann
© Mann
Wissenschaftlicher Mitarbeiter
NameDr. Claudio Frazão
Synthetische Biotechnologie und Systembiologie
Eine verschlüsselte E-Mail über das SecureMail-Portal versenden (nur für TUD-externe Personen).
Project funding:
DFG – Deutsche Forschungsgemeinschaft
Project duration:
01.03.2025-29.02.2028
Contact: