Research Group Synthetic Biotechnology and Systems Biology
Classical metabolic engineering consists of switching off and amplifying individual reactions in a targeted manner in metabolic networks of production strains in order to optimize the biosynthesis of a target molecule. Although considerable success can be achieved using this technology, the range of molecules that can be produced in this way is limited to natural metabolic products.

© Schmidt

© Schmidt

© Schneider
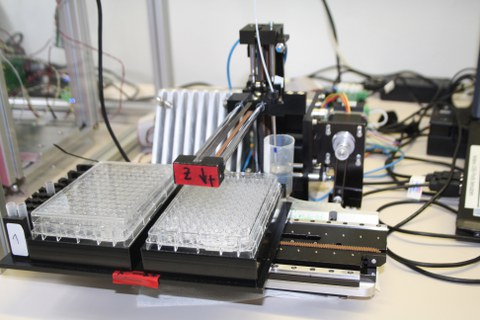
© Schneider

© Schneider

© Schneider
In order to expand the range of microbially usable substrates and producible products, we design synthetic metabolic pathways and implement them by engineering enzymes and production strains in a targeted manner.
A main focus of our work is the establishment of synthetic metabolic pathways for the utilization of green ethylene glycol (EG), which can be produced (electro)chemically fromCO2 or by hydrogenolysis of lignocelluloses. Compared to other microbial substrates that can be derived fromCO2, such as methane, synthesis gas or methanol, EG has a number of technical process advantages. These lie in particular in the avoidance of the phase transition of the substrate, the low oxygen demand of EG assimilation and an advantageous stoichiometry of the synthetic metabolic pathways for the production of even-numbered molecules. Overall, this technology should make the use ofCO2 and biomass in the circular economy more attractive.
In addition, we are developing enzyme systems for the cell-free regeneration of ATP from low-cost basic chemicals. These systems can be coupled with a variety of ATP-dependent enzymatic product syntheses and will be used, for example, in the field of cell-free protein expression.
In our work, we apply modern computational methods (flux balance analysis, modeling of enzyme structures) and experimental techniques(13C-basedmetabolic flux analysis, metabolome analysis, rational and evolutionary enzyme design) to efficiently realize the implementation of metabolic pathways and strain development.
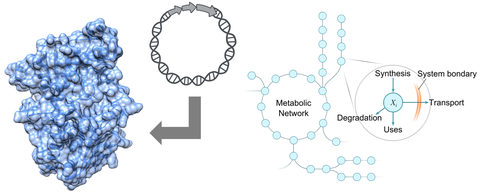
3D-Modell eines Enzyms und metabolisches Netzwerk
Focus areas:
- Synthetic metabolic pathways for the utilization of ethylene glycol (derived fromCO2 or lignocellulose) for microbial product syntheses
- Systems biology characterization of production strains and cell systems
- Development of ATP-regenerating enzyme cascades for cell-free reaction systems
- ESFplus junior research group CARBONCYCLE
Projects of the Bioprocess Engineering research group:
All research projects of the Chair of Bioprocess Engineering
- ScampiLys - Production of lysine from shrimp waste (cooperation with Hanoi University of Science and Technology, funded by BMBF)
- SynBioMet - Use of synthetic biology for the production of 2,4-dihydroxybutyrate (cooperation with ESPCI Paris, INSA Toulouse, Adisseo, funded by SAB)
- Enzyme cascades for cell-free ATP regeneration for the synthesis of valuable substances (funded by ZIM and DFG, cooperation with c-LEcta and University of Hamburg)
- Development of a synthetic metabolic pathway for the carbon-conserving production of acetyl-CoA from ethylene glycol (funded by the DFG)
- Enzyme cascades for cell-free ATP regeneration for the synthesis of valuable substances (funded by ZIM, cooperation with c-LEcta)
- CoBioMetal - Combined biotechnological and electrochemical wastewater valorization for metal recovery. Sub-project: Systems biology study of fungal strains during metal recovery from process wastewater (funded by the SAB, cooperation with TU Bergakademie Freiberg)
- KoSyn - Controlled synergy: Peptide-controlled cell-cell communication of yeasts and bacteria in (bio)technological processes (funded by the ESF, cooperation between the Chairs of General Genetics, General Microbiology, Hydrochemistry, all TU Dresden)
Research group leader:
 © Ritz
© Ritz
Prof. Dr.-Ing. Thomas Walther
Send encrypted email via the SecureMail portal (for TUD external users only).
Scientists of the research group:
 © Mann
© Mann
Project researcher
NameDr. Claudio Frazão
Synthetic Biotechnology and Systems Biology
Send encrypted email via the SecureMail portal (for TUD external users only).

Wissenschaftliche Mitarbeiterin
NameMs Laura Lilienthal M. Sc.
Synthetische Biotechnologie und Systembiologie
Send encrypted email via the SecureMail portal (for TUD external users only).
 © Mann
© Mann
Project researcher
NameM.Sc. Kenny Rabe
Synthetic Biotechnology and Systems Biology
Send encrypted email via the SecureMail portal (for TUD external users only).

wissenschaftlicher Mitarbeiter
NameMr Johannes Radde M.Sc.
Send encrypted email via the SecureMail portal (for TUD external users only).

Research associate
NameDipl.-Ing. Elly Straube
Synthetic biology and systems biology
Send encrypted email via the SecureMail portal (for TUD external users only).

Wissenschaftlicher Mitarbeiter
NameMr Alrik Titze Dipl.-Ing.
Synthetische Biologie und Systembiologie
Send encrypted email via the SecureMail portal (for TUD external users only).






