TOPIC NEURAL STEM CELLS
In neurobiology, the previous dogma on the unchangeability of the adult mammalian brain and its inability to give rise to new neurons has been challenged since the early nineties. Generally, it is now accepted that neurogenesis persists in adults to support life-long learning and brain repair after injuries. Though the debate continues whether neurogenesis occurs in humans throughout life one wonders why the Homo sapiens may differ from most other species.
Neurogenesis
Neurogenesis occurs in two major germinal niches in the adult mammalian brain, namely, the subventricular zone (SVZ) or periventricular niche, which is located below the ependyma of the lateral wall of the lateral ventricles, as well as the subgranular zone (SGZ) in the dentate gyrus of the hippocampus. The primary progenitor cells or neural stem cells (NSCs) in these regions display astroglial properties and are derived from radial glia cells, which function as NSCs during embryonic development but persist until adulthood. Slowly proliferating stem cells give rise to transit amplifying precursor cells, which eventually form young neurons.
Projects
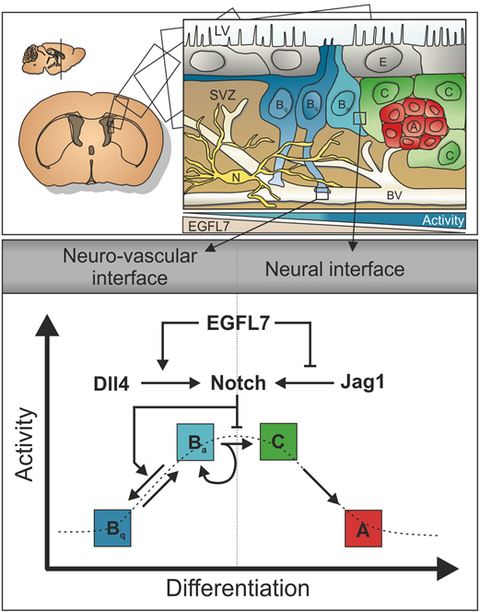
One of the most prominent molecular regulators governing neurogenesis is Notch, a master regulator of cell fate, since the self-renewal potential of NSCs relies on intact Notch signaling mediated by the binding of the receptor Notch1 to its canonical ligands Jagged1 and Dll4. We identified the protein EGFL7 as an inhibitor of Jagged-induced Notch signaling but stimulator of blood vessel-derived Dll4 and therefore as a key regulator. Obviously, the role of Notch signaling in NSCs and neurogenesis is in the center of our attention.
Within the framework of the CRC1080, we study the pivotal role of EGFL7 in adult neurogenesis and the consequences for behavior, learning and memory in the hippocampus. Further, we analyze how the connectivity and synaptic plasticity of adult-born neurons are impaired upon the loss of EGFL7 and how this affects learning and memory.
Anatomically, neurogenesis often takes place in the neurovascular niche, which is formed by endfoot connections of NSCs and local blood vessels. Various vessel-derived factors have been identified that affect neurogenesis on a molecular level. The existence of angioneurins, i.e., proteins that fulfil functions in neurogenesis and angiogenesis, is particularly interesting and dates back to the days of Andreas Vesalius, the founder of modern anatomy, who described the similarities among neural and vascular networks as early as in the 16th century.
Clinical relevance
Beyond its biological significance the phenomenon of adult neurogenesis also possesses a clinical dimension. Since the initial description of NSCs, researchers have wondered about the potential of the brain to repair itself and about the use of neural progenitor cells as therapeutics to treat brain damage such as ischemic stroke. Although in the meantime a general consensus has been reached that under physiological conditions the contribution of NSCs to brain repair is qualitative but not quantitative, there is evidence that ischemic brain injury leads to the recruitment of neural progenitor cells and the repair of local neuronal tissue. How pathological conditions such as stroke or Alzheimer’s activate the NSC pool and whether this leads to a local repair is in the center of our attention.
Most relevant papers
- Frank Bicker F ... Schmidt MHH (2017) Neurovascular EGFL7 regulates subventricular neural stem cells, neurogenesis and olfactory perception. Nature Comm 8, 15922. (Impact Factor 12.4)
- Schmidt MHH et al. (2009) Epidermal growth factor-like domain 7 (EGFL7) modulates Notch signalling and affects neural stem cell renewal. Nat Cell Biol 11 (7), 873-880. (Impact Factor 19.5)