Sep 21, 2021
Researchers overcome translational gap by developing novel in vivo model for the labeling of insulin secretory granule pools
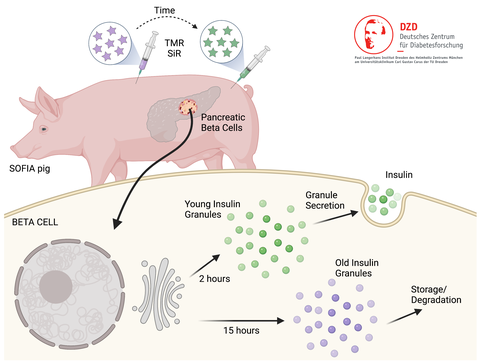
Pancreatic beta cells produce, store and secrete insulin upon elevated blood glucose levels. However, the highly regulated process of insulin secretion, especially its molecular features and the stimuli behind this process have not yet been fully understood. Furthermore, the current understanding of beta cell function is mostly derived from studies of ex vivo isolated islets in rodent models. To overcome this translational gap and to study insulin secretory granule turnover in vivo, a national research team under the lead of scientists from the Paul Langerhans Institute of the Helmholtz Zentrum München at the University Hospital Carl Gustav Carus and Faculty of Medicine of TU Dresden (PLID) and the LMU Munich has generated a transgenic pig model allowing for the first time the in vivo fluorescent labeling of age-distinct insulin secretory granule pools, hence providing a close-to-life readout of insulin turnover in normoglycemic conditions. The results of this highly collaborative project have been published in the renowned journal `Proceedings of the National Academy of Sciences of the United States of America (PNAS)`, given the likelihood that this novel large animal model close to humans will provide insight into the turnover of insulin pools in physiological and pathological conditions resembling human type 2 diabetes.
Dysfunction of pancreatic beta cells is a key contributor to type 2 diabetes (T2D), a disease affecting several hundred million people worldwide. Each beta cell stores insulin in several thousand organelles termed insulin secretory granules (SG), which release insulin extracellularly when blood glucose is elevated, as following a meal. Remarkably, only a small fraction of the insulin SG is being released and preferentially those that have been more recently produced. So far, however, our understanding of the principles governing insulin SG turnover remains very rudimental and derives exclusively from ex vivo experiments using isolated pancreatic islets from e.g., genetically modified mouse models or in vitro work with insulin-producing cell lines. Recently, transgenic pigs have been made available that allow for conducting beta cell research in a context even closer to humans.
In this new study, the researchers describe the generation and characterization of a transgenic pig model called SOFIA (Study OF Insulin granule Aging). “The first step in the generation process was the fusion of a SNAP-tag to the insulin gene, which allows the conditional and flexible labeling of age-defined insulin SG pools with fluorophores of different colors over longer time spans”, explains Dr. Elisabeth Kemter from the Wolf lab at the LMU Gene Center, one of first coauthors of the study. “Next, we had to transfect this vector into pig cells. These were used for somatic cell nuclear transfer to produce embryos which were subsequently transferred into estrus-synchronized gilts. Afterwards, we identified the SOFIA pig with the highest INS-SNAP expression rate in the beta cells, which was subsequently used to set up the pig line for the following further experiments.”
By using immunostaining and confocal microscopy for the SNAP-tag and Insulin the scientists showed colocalization of SNAP with insulin in the islets of Langerhans and determined the transgene integration site by targeted locus amplification (TLA) with subsequent next generation sequencing (NGS). “We located the transgene at a single locus in a non-coding region on chromosome 11 and further characterized the INS-SNAP expression levels and localization on cellular level”, says Dr. Andreas Müller from the Solimena lab at the PLID. “Overall, the ultrastructure of the beta cells of SOFIA pigs was comparable to that of pigs not expressing insulin-SNAP and the insulin SGs, mitochondria and endoplasmic reticulum appeared normal without any signs of stress or structural alterations. And importantly, glucose tolerance tests revealed no obvious difference in the blood glucose clearance of SOFIA in comparison to wild-type pigs and also the plasma insulin levels were comparable between the two groups. We could then show that the sequential application of two SNAP-dyes of different wavelengths resulted in insulin SG pools of different colors. Ultimately, our system enables us to follow insulin turnover in a close-to-life setting.”
With this successfully generated and characterized transgenic pig, the research team has taken a significant step toward overcoming two major limitations of current beta cell research: the translational gap between rodents and humans and ex vivo experiments for insulin turnover using isolated pancreatic islets. Especially the first point is of relevance, as the transgenic pig model is closing this translational gap, because, i) pigs are akin to humans in the anatomy of their gastrointestinal tract and the morphology and function of the pancreas, ii) it has been demonstrated that the structure and composition of pig pancreatic islets is much closer to human than to rodent islets and iii) the molecular and developmental signatures of pig and human islets / beta cells are also more similar to each other than those of human and rodents.
Taken together, the pig is a compelling model organism not only for the study of systemic metabolism, but specifically also for that of islets and beta cell function. Future studies will now aim at the optimization of the delivery of the labeling dye to the beta cells and the marking of insulin SG produced at different times. Ultimately, the SOFIA pig will be crossed to other available diabetic pig models to gain insights into the changes in insulin SG turnover in T2D.
Original Publication:
Elisabeth Kemter, Andreas Müller, Martin Neukam, Anna Ivanova, Nikolai Klymiuk, Simone Renner, Kaiyuan Yang, Johannes Broichhagen, Mayuko Kurome, Valeri Zakhartchenko, Barbara Kessler, Klaus-Peter Knoch, Marc Bickle, Barbara Ludwig, Kai Johnsson, Heiko Lickert, Thomas Kurth, Eckhard Wolf, Michele Solimena
Sequential in vivo labeling of insulin secretory granule pools in INS-SNAP transgenic pigs
doi: https://doi.org/10.1073/pnas.2107665118
Link: https://www.pnas.org/content/118/37/e2107665118
Contact:
Prof. Dr. Dr. Michele Solimena
Molecular Diabetology
DZD-Paul Langerhans Institute of the Helmholtz Zentrum München at the University Hospital and Faculty of Medicine Carl Gustav Carus of TU Dresden
Email: