Forschungsüberblick
The Mueller-Planitz lab studies the core components of chromatin – the nucleosomes and the machinery that places them in the genome. Nucleosomes are crucial to human health. Aging, for instance, disrupts the nucleosome landscape, destabilizing the genome, and mutations in nucleosomes are drivers of cancers. Nucleosomes serve both as barriers that restrict access to the genome and as a medium to accumulate epigenetic marks. Correspondingly, the locations of nucleosomes in the genome are precisely controlled by so called nucleosome remodeling complexes. Remodelers move, assemble, or eject nucleosomes in an ATP-dependent fashion. Some also even the spacing between nucleosomes, setting a characteristic nucleosome-to-nucleosome distance. These ‘spacing remodelers’ thereby generate arrays of nucleosomes with a surprising regularity, and these arrays are conserved throughout eukaryotes. Their function however remains elusive.
The overarching aim of the Mueller-Planitz lab is to elucidate the biogenesis of the nucleosome landscape and dissect its biological function under physiological and pathological conditions. To achieve this goal, his lab bridges methodologies of molecular biology, genetics, genomics, biophysics, structural biology, and enzymology. They develop cutting-edge technology to visualize individual nucleosome patterns in single cells, to deduce systems-level properties of tens of thousands of nucleosomes in cells, and to dissect the mechanism of nucleosome remodeling genome-wide in vivo and in vitro.
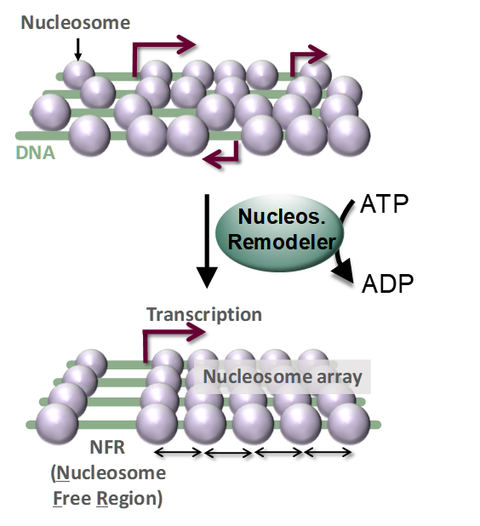
Remodeling enzymes set up the canonical nucleosome organization of genes. In an ATP hydrolysis-dependent manner, remodelers open up a ‘nucleosome free region’ (NFR) and generate an array of nucleosomes with even spacing downstream of the transcription start site (arrow; bottom). Without remodelers (top), cryptic promoters open up leading to spurious transcription.
Future projects and goals
· Changes in chromatin during natural and premature aging
· Transcription through chromatin
· Conformational dynamics of nucleosome remodelers
· Chromatin remodeling in phase-separated chromatin
· Biogenesis and maintenance of heterochromatin