Research Focus
Prof. Ünal Coskun is the founder and head of the Center of membrane biochemistry and lipid research (ZML). He heads the group of Membrane Biochemistry.
The starting point of my new research interest were two fundamental questions:
- Why do eucaryotic organisms invest that much energy into synthetizing a dazzling array of lipids, when only one lipid is sufficient to form a functional membrane bilayer?
- And, if proteins and lipids have co-evolved, what are the consequences of specific protein-lipid interactions for protein regulation as well as membrane targeting of proteins?
Understanding the bioactivity of lipids on protein structure and function is an emerging field that is expected to make pivotal contributions to our understanding of important cell biological processes (Coskun and Simons, 2011, Structure). The overarching theme of my research group is “Membrane Biochemistry of Cell Signaling”. We challenge the mutual interdependence of lipid-protein interactions by an interdisciplinary approach, combining cell biology and synthetic biology as well as protein biochemistry, structure biology and biophysics. Our strong expertise allows us study lipid-protein interaction based phenomena at different scales, from the organ and cellular systems down to minimal synthetic systems in which we can control the proteins as well as the lipid, for instance to monitor the allosteric effects of specific lipids on fundamental receptors such as the EGF receptor (Coskun et al., 2011, PNAS) and the insulin receptor.
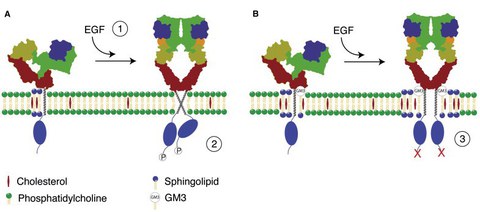
Proposed allosteric inhibition scheme of EGF receptor kinase activation by the ganglioside GM3 (A) EGF receptor association with sphingolipid/cholesterol domains prevents aberrant activation of EGFR signaling. EGF ligand binding promotes EGFR dimerization (1) leading to the formation of an active dimer (2). (B) When GM3 is present in the bilayer, the direct association of GM3 with the EGFR ectodomain leads to the inactivation of the EGFR kinase activity (3). For details please read Coskun and Simons (2011) Structure © Coskun
Working at different scales also allows us to focus on understanding the pathology of diseases, such as diabetes, from the membrane biochemical point of view.
A key unanswered question in diabetes research is is how β-cells choose between proliferation and insulin secretory granule (ISG) production. Moreover it remains unclear how β-cells can generate a graded response to insulin or even remain sensitive to it, conceivably being exposed to far greater concentrations of the hormone than other cell types such as adipocytes or muscle cells. A part of the explanation might be the changes in plasma membrane lipid composition that accompany the secretion process and lead to changes in signaling properties of the receptors present at the plasma membrane (IGF-1R, IR). Could lipid changes in the plasma membrane of secretory β-cells be responsible for modulating the cellular response to insulin exposure?