Large-scale extracellular matrix architecture and tissue-like morphogenesis as emerging properties of bacterial multicellularity
- Prof Dr Regine Hengge Humboldt-Universität zu Berlin, Institut für Biologie/Mikrobiologie, Arbeitsgruppe Mikrobiologie.
The project aims at thoroughly understanding key architectural, functional and regulatory properties that emerge from bacterial multicellularity using E. coli macrocolony biofilms as a model system.
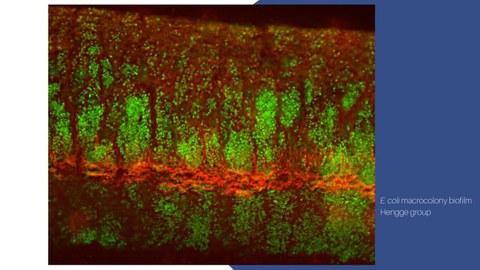
Bacterial biofilms are extracellular matrix (ECM)-embedded multicellular aggregates that show spatio-temporal self-organisation by generating and reacting to primary and secondary metabolic gradients. These are translated into a spatially distinct, reproducible three-dimensional ECM architecture that is >2 orders of magnitude larger than the millions of cells that coordinately build it.
ECM composition and specific architecture are a prerequisite for the emerging properties of this multicellular bacterial life form: (i) the elastic tissue-like buckling and folding of very flat macrocolonies into patterns of ridges or wrinkles without breakage, i.e. macroscopic morphogenesis; (ii) the potential for homeostasis and ‘niche construction‘ in the extracellular, yet biofilm-internal space; and (iii) protection against microbial predators. As the latter two properties reduce maintenance energy within the biofilm population and thus represent a fitness gain, ECM-associated multicellularity can be considered an emergent property of life itself.
Research Focus
Thus, the project aims at:
- Understand the formation of the biofilm-internal ECM architecture and how it relates to macroscopic morphogenesis.
- Detecting and demonstrating the formation and key molecular function of large-scale metabolic and regulatory gradients (nutrients, O2, fermentation products, c-di-GMP) in the physiological differentiation that underlies the formation of the ECM architecture.
- Clarifying the genetic-regulatory rewiring that allows for evolutionary plasticity of the ECM architecture and its consequences for macroscopic morphogenesis as observed with certain commensal and pathogenic E. coli strains.
Insights obtained from this study with the genetically highly tractable model organism E. coli will be fundamental to understanding the evolutionary transition from the single cell state of bacteria and other microbes to early functional multicellularity.
| Researcher |
| Dr Katharina Preßler (Postdoc) |
Contact US
Humboldt University of Berlin
Email regine.hengge@hu-berlin.de
Humboldt University of Berlin
Email presslek@hu-berlin.de