Festkörper-NMR-Spektroskopie
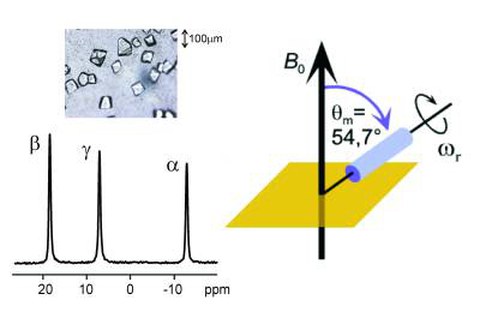
Aufgrund der methodischen und apparativen Fortschritte der vergangenen Jahre gewinnt die Festkörper-NMR-Spektroskopie derzeit zunehmend an Bedeutung in der Biologie. Die Entwicklung von supraleitenden Hochfeldmagneten (derzeit bis zu ca. 25 T für Magnete mit weiter Bohrung) und von MAS-Turbinen (MAS: magic angle spinning) mit Probenrotationsfrequenzen von bis zu über 100 kHz Probenrotationsfrequenz sind wichtige Gründe für diesen Erfolg. Das Prinzip der MAS-Technik wird im oberen Bild illustriert, zusammen mit einem hochaufgelösten P-31 {H-1} Kreuzpolarisations-MAS NMR-Spektrum eines Nukleotids (GppCH2p), welches an ein Protein (Ras) gebunden ist.
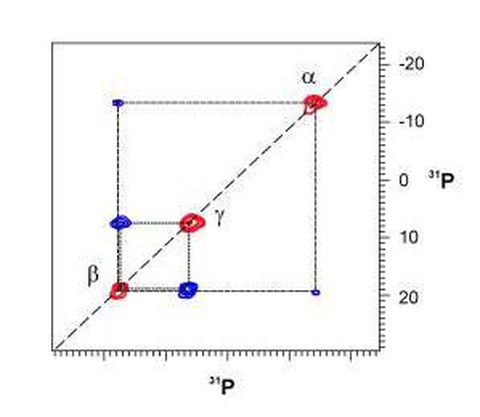
Spektrale Auflösung und Empfindlichkeit moderner Festkörper-NMR-Experimente sind inzwischen ausreichend um mehrdimensionale NMR-Techniken, wie sie in der biomolekularen Flüssigkeits-NMR-Spektroskopie längst üblich sind, auch auf feste Proteine anzuwenden. Die Abbildung zeigt ein zweidimensionales H-1-getriebenes P-31-P-31 Spindiffusionsspektrum eines Nukleotids (GppCH2p), welches an ein Protein (Ras) gebunden ist.
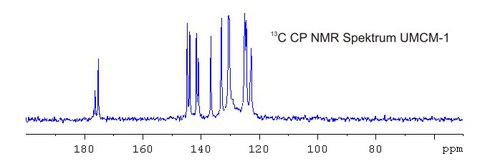
Mit Hilfe der 13C-FK-NMR-Spektroskopie kann beispielsweise die Gerüststruktur von MOF´s sowie dynamische Verhaltensweisen einzelner Strukturgruppen charakterisiert werden.
Unsere Arbeitsgruppe verfügt über zwei moderne 800 MHz und 300 MHz Festkörper-NMR-Spektrometer mit drei Frequenzkanälen.