Nov 20, 2020
One-Pot Synthesis of Boron-Doped Polycyclic Aromatic Hydrocarbons via 1,4-Boron Migration
Boron-doped polycyclic aromatic hydrocarbons (B-PAHs) are of great current interest due to their excellent photophysical properties and their promising applications in organic field-effect transistors (OFETs) and organic light-emitting diodes (OLEDs). Although significant efforts have been made in the past few years, the modular synthetic routes toward B-PAHs, particularly for dual-B doped PAHs, remain limited due to the intrinsic instability of B-PAHs against moisture and oxygen. So far, the existing methods for the synthesis of B-PAHs usually involve special precursor design or multiple synthetic steps, e.g., an additional oxidation etc., resulting in low overall yields of synthetic procedures. Thereby, it is highly attractive to develop cost-effective methodologies for the synthesis of B-PAHs from the readily available starting compounds.
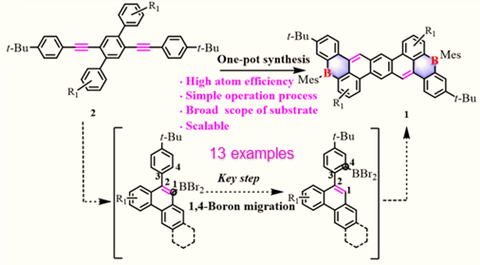
Recently, researchers from Technische Universität Dresden (TUD, Chair of Molecular Functional Materials) and collaborators (HKU, Hong Kong) reported a novel one-pot synthetic method toward a series of mono/dual B-PAHs (1a-1o) via an unprecedented 1,4-boron migration process from readily available ortho-aryl substituted arylalkynes. This protocol renders a broad scope of substrates (13 examples) with high atom efficiency and functional group tolerance. The reaction mechanism involves a sequence of borylative cyclization, 1,4-boron migration, and electrophilic C-H borylation. Notably, this is the first example regarding the 1,4-boron migration in the π-conjugated system. Single-crystal X-ray analysis of the represented compounds 1b and 1k demonstrate that the boron atom locates at the zigzag edge and adopts a trigonal planar geometry. The achieved B-PAHs 1a-1o display excellent fluorescence with the photoluminescence (PL) quantum yields (ΦPL) up to 97% in dichloromethane solution (1g) and 91% in the solid-state thin film (1f). As a proof-of-concept study, blue and green OLEDs are fabricated based on B-PAHs 1f and 1k with an external quantum efficiency (EQE) of 3.5% and 3.2%, respectively, demonstrating their promising applications in organic optoelectronic devices.
Reference: Jin-Jiang Zhang, Man Chung Tang, Yubin Fu, Kam-Hung Low, Ji Ma, Lin Yang, Jan J. Weigand, Junzhi Liu,*, Vivian Wing-Wah Yam, Xinliang Feng*.
Acknowledgements: This work was financially supported by the European Union’s Horizon 2020 research and innovation programme (GrapheneCore3 No. 881603, Marie Skłodowska-Curie grant No 813036) ERC T2DCP, the German Research Foundation (DFG) within the Cluster of Excellence “Center for Advancing Electronics Dresden (cfaed)” and DFG-NSFC Joint Sino-German Research Project (EnhanceNano), as well as the DFG-SNSF Joint Switzerland-German Research Project (EnhanceTopo). We thank the Center for Information Services and High Performance Computing (ZIH) at TU Dresden for generous allocations of compute resources. J. Liu is grateful for the startup funding from The University of Hong Kong and the funding support from ITC to the SKL.