Organic 2D Materials
The discovery of graphene in 2004 has triggered enormous interest in developing chemistry of synthetic two-dimensional materials (2DMs). In this O2D group, we mainly focus on the development of organic 2DMs, which refer to crystalline 2D nanostructures comprising carbon-rich repeated units linked by covalent/noncovalent bonds, including synthetic graphene, 2D polymer (2DP), 2D supramolecular polymer (2DSP), single/few-layer 2D COFs/MOFs, etc. We expect to establish novel chemical/synthetic methodologies toward the controlled synthesis of organic 2DMs and achieve delineation of reliable structure-property relationships and superior physical and chemical performances of organic 2DMs. In this respect, four research areas are involved, including (1) Development of interface-assisted synthesis methodologies; (2) Synthetic 2D polymers/2D COFs; (3) MOFtronics: chemistry of 2D conjugated MOFs and functions for (opto-)electronics, spintronics and energy; (4) Novel van der Waals heterostructures.
Research Area I: Development of Interface-Assisted Synthesis Methodologies
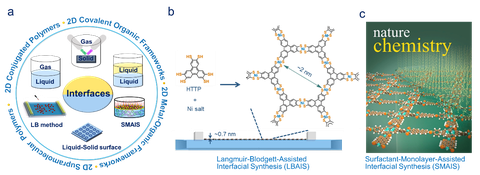
An interface is a flat or curved space (or a phase boundary) between two different matters, or two different phases in one matter. The thickness of the interface space can range from several angstroms to nanometer and even to micrometer size. In our work, we aim to develop interfaces as key roles in “bottom-up” synthesis, which is advantageous for directing the pre-orientation of the molecules or precursors for subsequent 2D polymerization toward organic 2DMs. Typically, the reactions at the interfaces between air−water (or gas−liquid, LB), liquid−liquid, liquid−solid, and gas−solid (or vacuum-solid) are explored for the 2D polymerization by linkage chemistry and the paramount control over the morphology and the structure of organic 2DMs.[1-3] In our latest work, we have established a surfactant-monolayer-assisted interfacial synthesis (SMAIS) method to prepare 2D polymers, like 2D polyimides,[4] 2D polyimines[5] and 2D boronate ester-linked polymers,[6] thus achieving few-layers and micrometer-sized single-crystalline domains.
Figure | (a) Schematic illustration of interface-assisted reactions toward organic 2DMs.[1] (b) Langmuir-Blodgett-assisted interfacial synthesis (LBAIS) toward monolayers.[2] (c) Surfactant-monolayer-assisted interfacial synthesis (SMAIS) toward few-layers.[4]
Research Area II: Synthetic 2D polymers/2D COFs
Two-dimensional polymers (2DPs) and their layer-stacked 2D covalent organic frameworks (2D COFs) have emerged as a class of structurally defined organic 2D materials with exotic physical and chemical properties. These porous crystalline polymers generally comprise repeated units linked via covalent bonds with long-range ordering in two distinct directions and have displayed diverse physical and chemical properties for broad functions in (opto-)electronics, spintronics, membrane, catalysis, and energy storage and conversion. Yet, controlled synthesis of 2DP and 2D COF single crystals still remains an immense challenge.
- Dynamic covalent reactions
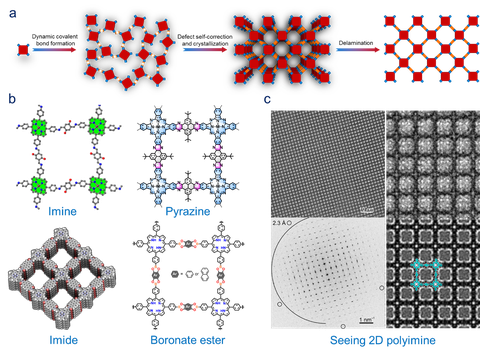
We aim to explore thermodynamically reversible dynamic covalent reactions (DCRs) for the synthesis of crystalline 2DPs/2D COFs at interfaces and in solution. DCRs allow the atomically precise integration of organic units to create periodic networks with long-range order linked by covalent bonds. A key feature of DCRs is the thermodynamically controlled product distribution at equilibrium. Typical examples of DCRs include the formation of boronate ester, imine, pyrazine, azine, imide, amide and azodioxy bonds. In our recent work, by combining DCRs and SMAIS, we successfully achieved few-layer 2DP single crystals with the domain size up to ~150 μm2.
Figure | (a) 2D polymerization under thermodynamic control relying on self-correction after bond formation toward layered crystals and subsequent delamination. (b) Representative 2D polymers/2D COFs based on imine,[5] pyrazine,[7] imide[4] and boronate ester[6] linkages. (c) HRTEM imaging and electronic diffraction of 2D polyimine by SMAIS.[8]
- Irreversible covalent reactions
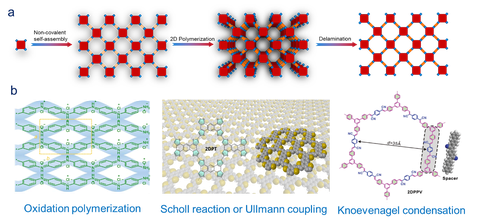
We aim to explore the utilization of kinetically irreversible covalent reactions (ICRs) for the synthesis of 2DPs and 2D COFs, which is highly attractive but remains challenging. To achieve the long-range order and layered crystals, 2D polymerization will be carried out under kinetic control relying on monomer pre-organization prior to bond formation. Typical ICRs including Knoevenagel,[9] Aldol-type and Horner-Wadsworth-Emmons condensation[10] reactions have been developed for the solvothermal synthesis of vinylene-linked 2D COFs, yielding fully conjugated polymer materials with high chemical and thermal stabilities. On the other hand, structurally-defined conjugated 2DPs can be realized via the on-surface Ullmann coupling under ultrahigh vacuum conditions. Recently, we also performed the oxidation polymerization on water surface by SMAIS and developed wafer-sized, highly crystalline quasi-2D polyaniline thin film,[11] which exhibited a high conductivity up to 160 S/cm.
Figure | (a) 2D polymerization under kinetic control relying on monomer pre-organization prior to bond formation toward layered crystals and subsequent delamination. (b) Representative examples: quasi-2D polyaniline via oxidation polymerization,[11] 2D polythiophene via Scholl reaction or Ullmann coupling, and 2D Poly-(phenylenevinylene) via Knoevenagel condensation reaction.[9]
- Functions for electronics, energy and membrane
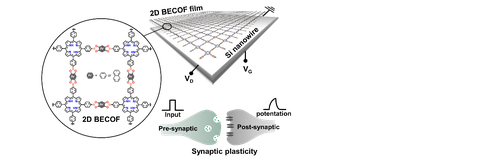
Synthetic 2D polymers and 2D COFs feature with intrinsic porosity and flexibility as well as tailored electroactivity and photoactivity. In our recent work, upon I2 doping, a phthalocyanine-based pyrazine-linked 2D COF exhibited high conductivity (10-3 S/cm) and record mobility reaching to 22 cm2/(Vs).[12] These unique features enable 2D polymers and 2D COFs broad functions in transistors, sensors, flexible devices, memory devices,[6] membrane separation, osmotic power generators, electro-/photo-catalysis, batteries and supercapacitors.[13]
Figure | Boronate ester based 2D polymer films for neuromorphic memory device.[6]
Research Area III: MOFtronics_chemistry of 2D conjugated MOFs and functions for (opto-)electronics, spintronics and energy
Two-dimensional (2D) π-conjugated metal-organic frameworks (2D c-MOFs) refer to layer-stacked MOFs linked by square-planar complexes with in-plane π-extended conjugation. They have similar structural features to graphite and other van der Waals layer-stacked materials, and in recent years have displayed much higher conductivities (up to 2500 S/cm) than conventional three-dimensional (3D) MOFs (<10-8 S/cm),[14] or demonstrated charge mobility of 220 cm2/Vs with band-like transport.[15] The enormous chemical and structural diversity of ligands and metal ions provides researchers with a playground filled with infinite creative possibilities, allowing design of 2D c-MOFs.[16] Although there are no commercial devices that yet incorporate 2D c-MOF materials, numerous proof-of-concept device demonstrations have appeared, such as transistors, photodetectors,[17] sensing, magnetics,[18,19] and energy storage[13,20,21] and conversion[22,23]. These advances are highly inspiring and demonstrate that 2D c-MOFs are an emergent class of layered electronic materials.
Figure | Schematic illustration of MOFtronics.[14,16]
- Chemistry of 2D conjugated MOFs
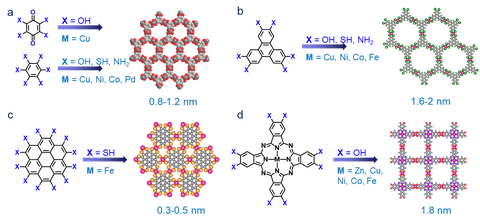
To date, around fifteen 2D c-MOFs have been reported based on planar polycyclic aromatic ligands (such as benzene, triphenylene, coronene, phthalocyanine and dibenzo[g,p]chrysene derivatives) with symmetrical functional groups (-OH, -NH2, -SH).[14,16] First of all, we aim to explore the influence of molecular design (type and geometry of ligand, metal) and material structure (redox-activity in linkage, layer stacking and arrangement) on the charge transport properties for 2D c-MOFs. Secondly, another essential question is how to optimize the reaction conditions to pursue high crystallinity.[24] Traditional approaches for controlling crystal growth generally involve varying solvent, concentration, reaction temperature, and time. Nevertheless, these strategies have failed in suppressing the anisotropic growth of a crystal because the in-plane metal-coordination bonds are too strong while the π-π stacking interaction is relatively weak. Thus, the crystal growth is usually dominated by the more reversible π-interaction to yield needle-like morphology with limited crystalline domains parallel to the intralayer direction, or even polycrystalline powders with tiny crystallites (up to hundreds of nanometers in size). Therefore, a rational ligand design will be the first step for establishing highly crystalline sample, thus achieving intrinsic electronic properties. On the other hand, we also explore a variety of synthetic methods to develop 2D c-MOFs, such as solvothermal synthesis, interface-assisted synthesis, on-surface synthesis and surfactant-assisted synthesis as well as CVD synthesis, which render 2D c-MOFs with various morphologies such as bulk crystals, ultra-thin nanosheets (<5 nm), and single-/few-layer films.
Figure | Representative organic ligands and the relevant 2D c-MOFs with hexagonal and square lattices.
- 2D c-MOFs for (opto-)electronics
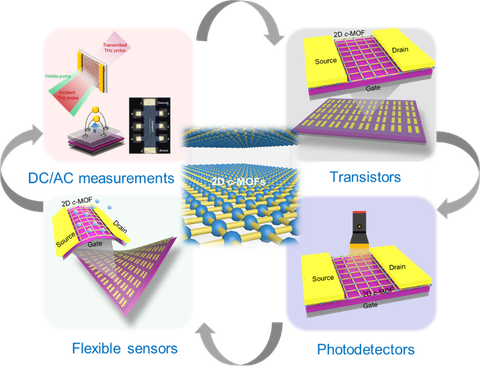
The high-conductivity or high-mobility 2D c-MOFs with tunable band structures exhibit promising potential as fascinating electroactive layers for opto-electronic devices. We are particularly interested in defining their intrinsic photo-/electron-transport properties via DC (like time-resolved terahertz spectroscopy) and AC measurements (like 2 probe, Hall effect and Van der Pauw).[15,17] As a consequence, we expect to establish a reliable structure-property relationship and achieve superior device performance for transistors, photodetectors and sensors.
Figure | Schematic illustration of 2D c-MOFs for (opto-)electronics.
- 2D c-MOFs for spintronics
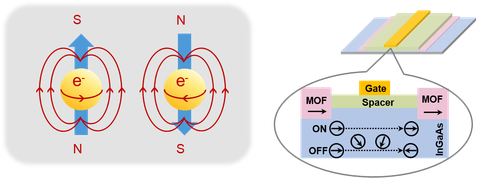
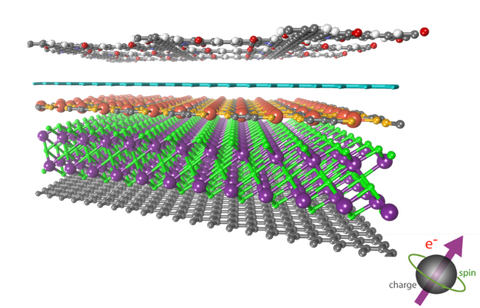
Spintronics, also known as spin transport electronics, has been intensely researched for the next generation of quantum computing and data storage, because spintronics could offer an exciting prospect of combining the semiconductor-based information operation (logic) and magnetic based data storage (memory) into the same device. Historically, spin-related phenomena have been widely studied in inorganic semiconductors; however, the entirely inorganic composition prevents a facile tunability of magnetic and conductive properties due to the lack of both ligand functionalization and structural diversity. In this respect, (semi-)conductive 2D c-MOF hold promise because the magnetic behavior can be tailored at the molecular scale through tuning their building blocks, metal centers (spin carriers) and even filling functional guest molecules or introducing radicals into the porous. For this purpose, we aim at the design and synthesis of 2D c-MOF magnets for spintronics.
Figure | Schematic illustration of 2D c-MOFs for spintronics.
- 2D c-MOFs for electrochemical energy storage
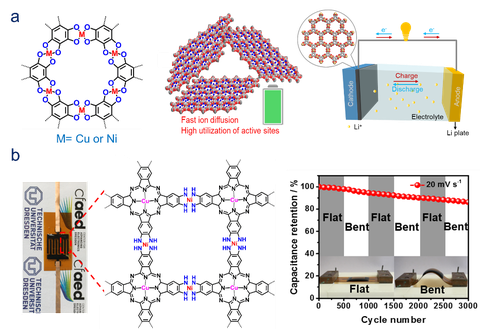
Conductive 2D c-MOFs incorporating redox active units have emerged as a novel class of multifunctional electrode materials for electrochemical energy storage. Metal-ion batteries and supercapacitors represent two major energy storage devices. Benefiting from the intrinsically conductive and porous properties, 2D c-MOFs have shown excellent performance in metal-ion batteries[21] and supercapacitors,[20] which could function as the power sources for the above mentioned MOFtronics.
Figure | Schematic illustration of 2D c-MOFs for electrochemical energy storage. (a) Li-ion battery.[21] (b) Flexible microsupercapacitor.[20]
- 2D c-MOFs for electrochemical energy conversion
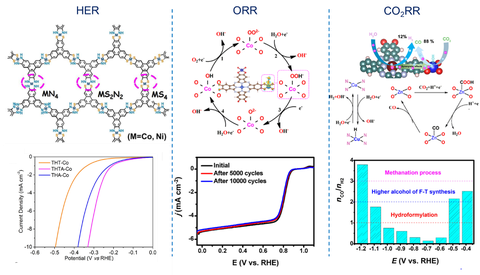
The exploration of effective electrocatalysts has advanced the development of electrochemical energy storage and conversion technologies, such as fuel cells, water splitting, and metal–air batteries. 2D c-MOFs are highly attractive catalytic systems due to the uniquely combining homogenous and heterogeneous features: 1) the incorporation of well-defined and highly active sites into a defined and stable scaffold ensures excellent catalytic activity and selectivity; 2) the porous metrics allow for sufficient and controllable mass transfer to and from the embedded active sites; 3) the molecularly defined catalytic environment around the active site allows for tuning the catalytic reaction by modifying the scaffold and facilitates deriving fundamental understanding of catalytic mechanism. To achieve high catalytic performance, our research focus on the design and synthesis of novel 2D c-MOFs with well-defined active sites and porous structures for mass transfer.
Figure | Schematic illustration of 2D c-MOFs for electrochemical energy conversion, including HER,[2,24] ORR22 and CO2RR.[23]
Research Area IV: Novel van der Waals heterostructures
Two-dimensional (2D) van der Waals heterostructures (vdWHs) are generated by integration of 2D materials with dangling-bond-free surfaces through the weak interlayer vdW interaction along the vertical direction. In recent years, 2D vdWHs have attracted increasing attentions due to the diverse components and novel functions in photodiodes, phototransistors, tunneling devices and memory devices. Through surface reconstruction and proximity effects between the neighboring layers, the optoelectrical properties of vdWHs can be tailored, thus tuning carrier density, improving electron-hole separation and accelerating charge transfer. In our work, we aim to establish bottom-up chemical methodologies toward the synthesis of organic 2DM based vdWHs, control the chemical synthesis for high-quality vdWH interface with atomic precision, and elucidate the structure and physical/chemical property of the synthetic vdWHs.
Figure | Schematic illustration of organic 2DM based vdWHs.
References:
- Chem. Rev. 2018, 118, 6189-6235.
- Angew. Chem. Int. Ed. 2015, 54, 12058-12063.
- Nat. Commun. 2016, 7, 13461.
- Nat. Chem. 2019, 11, 994-1000.
- Angew. Chem. Int. Ed. 2019, 59, 6028-6036.
- Angew. Chem. Int. Ed. 2020, 59, 8218-8224.
- J. Am. Chem. Soc. 2019, 141, 16810-16816.
- Sci. Adv. 2020, 6, eabb5976.
- Polym. Chem. 2016, 7, 4176.
- Angew. Chem. Int. Ed. 2020, 59, 23260-23625.
- Nat. Commun. 2019, 10, 4225.
- J. Am. Chem. Soc. 2020, 142, 21622-21627.
- J. Am. Chem. Soc. 2020, 142, 12903-12915.
- Chem. Rev. 2020, 120, 8581-8640.
- Nat. Mater. 2018, 17, 1027-1032.
- Chem. Soc. Rev. 2021, DOI: 10.1039/D0CS01160F.
- Adv. Mater. 2020, 32, 1907063.
- Nat. Commun. 2018, 9, 2637.
- Nat. Commun. 2019, 10, 3260.
- Adv. Funct. Mater. 2020, 30, 2002664.
- Chem. Sci. 2020, 11, 7665-7671.
- Angew. Chem. Int. Ed. 2019, 58, 10677-10682.
- Nat. Commun. 2020, 11, 1409.
- Nat. Mater. 2021, 20, 122-123.
- Chem. Eur. J. 2017, 23, 2255-2260.