DFG-Funded Project: KIF15 Regulation
Project number: 417890911 (view project at GEPRIS)
Project Summary
The capacity for eukaryotic cells to divide and move crucially depends on the microtubule cytoskeleton and its ability to be continuously reconfigured. During cell division, a micron-sized spindle-shaped microtubule structure drives the distribution of duplicated sister-chromatids into the emerging daughter cells. Mitotic spindle assembly and maintenance is governed by a set of so-called molecular motors (i.e., kinesins and dynein), small protein machines that consume chemical energy to transport cargo (in this case microtubules) throughout the cell by stepping along microtubule tracks.
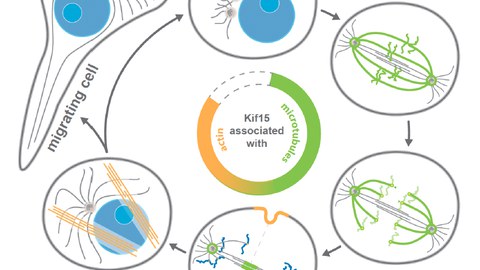
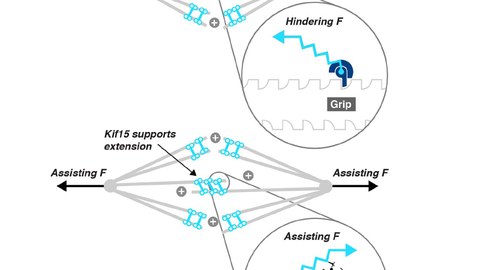
Spindle assembly starts with the separation of the spindle poles (i.e., the duplicated microtubule organising centres) driven by two functionally redundant tetrameric motors of the kinesin superfamily - kinesin-5 (EG5) and kinesin-12 (KIF15). Once the spindle has assembled, EG5 and KIF15 motors create extensile forces within the spindle, which are counter-balanced by the spindle-compressing activity of the dimeric kinesin-14 (HSET) and dynein – thereby maintaining a metastable microtubule structure. Initial spindle pole separation and spindle assembly crucially depends on Eg5 and loss of Eg5 activity cannot compensated by KIF15 unless the force equilibrium within the nascent spindle is shifted in favour of KIF15 (i.e., by KIF15 overexpression or by loss of dynein activity). Later in metaphase, KIF15 governs spindle maintenance, while EG5 activity becomes dispensable at this stage.
The precise duplication of the genetic information during S-Phase, as well as its faithful distribution into the emerging daughter cells during M-Phase are crucial to maintain a cell's identity during mitosis. Hence, motor redundancy during spindle assembly constitutes a crucial fail-safe mechansim that improves the robustness of cell division. Redundancy thus minimises the risk of mitotic failures or the occurrence of missegregated chromosomes, which are one cause for aneuploidy and thus for the subsequent transformation of 'normal' cells into malignant cancer cells. On the downside, however, motor redundancy poses a problem, when cells have already become malignant making it necessary to actually kill them.
Due to its central role in spindle assembly, current antiproliferative cancer treatment approaches aim to inhibit EG5 activity, thereby restricting tumour growth. However, cells chronically treated with EG5-inhibitors quickly acquire inhibitor resistance by upregulating KIF15 expression. In general, the overexpression of EG5 and KIF15 results in increased growth, survivability and invasiveness of tumour cells and is therefore associated with a bad prognosis for cancer patients.
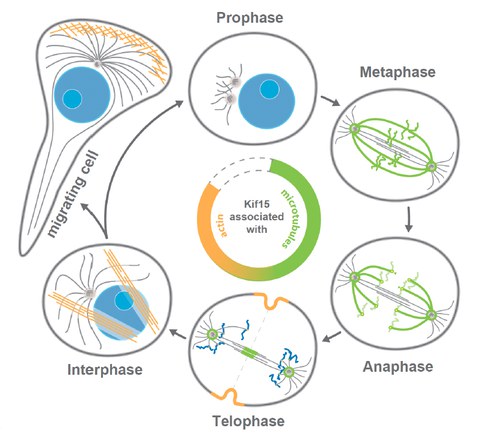
localisation of human KIF15 throughout the cell-cycle
While there is a broad agreement about the mechanism by which EG5 drives spindle assembly, the mechanistic role of KIF15 in this process is still under debate. This is in particular due to the fact that KIF15 motors are multifunctional and display many distinct cellular subpopulations (i.e., at the k-fibres, interpolar microtubules, chromosome arms and kinetochores). At the current state of research, however, it is not possible to link a particular motor function and/or cellular subpopulation of KIF15 to its role in mitotic spindle assembly. Yet, detailed mechanistic knowledge of KIF15 function is essential to understand its functional redundancy with EG5 during spindle assembly and thereby the emergence of EG5-inhibitor resistance in chronically treated cells. Interestingly, there is emerging evidence that KIF15 also has close ties with the actin cytoskeleton and may control cell migration. This aspect has been little studied so far but may have important implications for the metastatic behaviour of cancer cells overexpressing KIF15.
Dissecting the mechanics of KIF15 dependent spindle assembly, EG5-inhibitor resistance and cell migration control by linking cellular subpopulations and particular molecular functions of KIF15 to the respective mechanical processes.
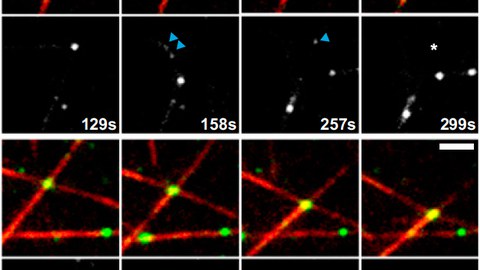
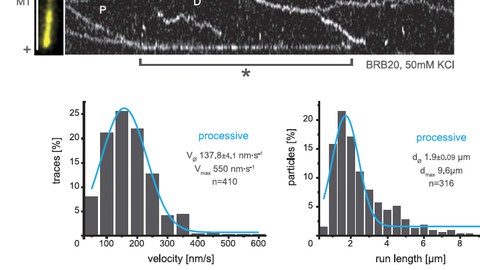
In a hypothesis-driven approach, we study KIF15 variants that carry either point mutations or short deletions within a newly mapped regulatory hub in the stalk of KIF15 that abolish either i) the motor’s ability to tetramerise, ii) its interaction with particular cellular subcompartments or iii) its association with the actin cytoskeleton. As certain motor functions depend on its oligomerisation state, we expect constitutively dimeric KIF15 (cdKIF15) variants to act as separation-of-function mutants. We analyse all KIF15 variants for their substructure localisation in vivo, their oligomerisation state and their ability to bind known interaction partners. Further, we characterise the biophysical and functional properties of these KIF15 variants in vitro and test them for their capacity to rescue spindle assembly in the absence of EG5 activity in vivo.
Project Team Members
Dr. Hauke Drechsler
Independent Project Leader
Tel. +49 (351) 463 43014