Dec 19, 2023
A Temporary Tug-Of-War: A Minimal System Unlocks Cellular Transport Secrets
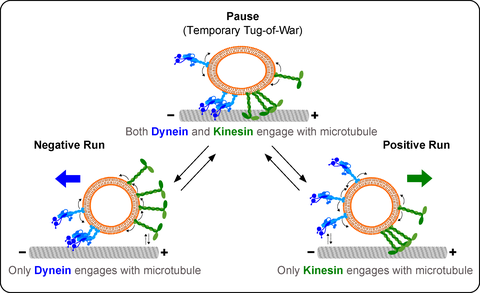
A visual representation of the findings. The net number of cargo-bound kinesin or dynein motor proteins engaging with the microtubule track decides whether it is moved in positive or negative direction. When both, kinesins and dyneins engage with the microtubule, the transport is often interrupted. Occasionally, the cargo is being pulled by motor proteins in opposite directions before one finally wins.
Cells are busy conglomerates. Different molecules and organelles have to be delivered to different locations at a specific time. How exactly are they reaching their destinations is a long-standing question in biology. Researchers from the Diez group at the B CUBE – Center for Molecular Bioengineering of TUD Dresden University of Technology and the Santen group at the Center for Biophysics at the Saarland University have now built a minimal version of a cell transport system outside a cell. Using the minimal system, the team discovered the principles of how cells control the direction of transport. The new study was published in the journal Nature Communications.
Cells are like busy factories. They need to transport molecules and organelles (cargo) reliably to different destinations within the cell. Defects in cellular transport have been associated with many diseases including Alzheimer’s, Parkinson’s, and Huntington’s.
The transport relies on a system of cellular train tracks known as microtubules. Two types of motor proteins, kinesin and dynein, can move in opposite directions along the microtubules to carry the cargo to its destination.
At any given time the cargo is attached to multiple copies of kinesin and dynein. Yet, it moves in only one direction. It is unclear what determines the moving direction.
“Cellular transport is a complicated system with many components. The microtubules themselves are a complex lattice of proteins that can already have influence over the transport of cargo. On top of that, there are many proteins bound to microtubules or to the cargo that might regulate the transport. Finally, there is the varying number of kinesins and dyneins at any given time,” explains Prof. Stefan Diez, research group leader at the B CUBE. “Given the complexity of transport inside the cell, we decided to take this system out of the cell, build a minimal version of it, and test different variables separately.”
A Bottom-Up Approach
The team led by Prof. Diez has successfully built an intracellular transport system outside the cell using purified components. Such a minimal system, consisting of microtubules, kinesin and dynein motors, and a phospholipid cargo, was sufficient to reproduce key features of transport in the cell.
“Other groups have tried to reconstitute intracellular transport in-vitro. So far, most of the other systems remained stationary or moved the cargo at speeds much slower than those observed in the cells. Our system is different. It works very much like the native transport in the cell with fast runs of cargo in either direction, sporadic pauses in between the movements, and changes in direction – the features that are characteristic to transport within the cell,” says Dr. Rahul Grover, a scientist in the Diez group and one of the authors of the study.
A Balance Between the Motors
Having a minimal system, the team used it to address what controls the direction of cargo transport. “If the cargo was bound only to kinesin or dynein, we would expect it to move in only one direction. However, in reality, it is always bound to multiple copies of kinesin and dynein. Yet, it moves in only one direction with frequent short interruptions and sometimes reverses its direction,” says Dr. Ashwin D’Souza, a former doctoral student in the Diez group and one of the authors of the study.
The group found that the number and type of motor proteins influenced the direction of the transport. The net number of cargo-bound kinesins vs. dyneins interacting with the microtubules would decide whether it moved in one direction or the other. “However, what baffled us though was the fact that the number of opposing motor proteins did not influence the speed of transport,” says Prof. Diez. When it moved, the cargo always moved as if the opposing motors were not present.
A Temporary Tug-of-War
The presence of opposing motors had some other distinct influence on the transport though. When bound to both kinesins and dyneins, the transport was often interrupted either to instantaneously change the direction or to pause the movement whatsoever.
“We looked in more detail at what happens during such pauses. Occasionally, the cargo would elongate before reversing the direction of its movement. It looked like both motors were pulling on it in opposite directions before one finally won. A true tug-of-war at the molecular scale,” explains Dr. Grover.
The Diez group teamed up with the Santen group at the Center for Biophysics at the Saarland University who are experts in theoretical modeling and simulations. Using the results from the minimal system, the Santen group was able to provide numerical simulations that gave additional insights into the decision-making process behind the direction of transport.
Using computer simulations, the Santen group could identify different motor configurations that can lead to reversals of moving direction. They found that the pauses and changes of direction do not have to be regulated by any additional factors. These changes can result from low numbers of attached motor proteins, with motor proteins randomly attaching to and detaching from the microtubule at all times.
The Future of the Minimal System
The reconstituted system opens the door to exploring other intriguing questions about intracellular transport. “We are interested to see what is the influence of environmental factors such as temperature and viscosity on the system. We also plan to introduce more variables into the system, e.g., proteins that bind to microtubules, and see whether they would - acting as molecular roadblocks - influence one type of motor more than the other. If so, they might regulate the direction of transport, e.g., in neuronal cells where hindered and misguided transport often leads to pathological conditions ,” says Prof. Diez.
The collaborative study was funded by the German Research Foundation (DFG) and the German Federal Ministry of Education and Research (BMBF). The work would not be possible without the support of the Technology Platform at the Center for Molecular and Cellular Bioengineering of TU Dresden and specifically the Molecular Imaging and Manipulation Facility led by Dr. Jens Ehrig.
Original Publication
Ashwin I. D’Souza, Rahul Grover, Gina A. Monzon, Ludger Santen & Stefan Diez: Vesicles driven by dynein and kinesin exhibit directional reversals without regulators. Nature Communications (November 2023)
Link: https://doi.org/10.1038/s41467-023-42605-8
