Jun 05, 2025
Breaking the Cycle: Researchers Develop New Small Molecules to Disrupt Cancer Growth
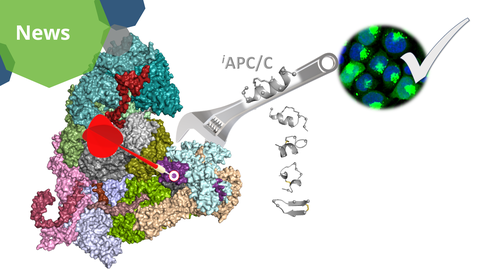
A visualization of the study results. The APC/C inhibitors target the defined region of the Anaphase Promoting Complex (APC/C) to stop cells from dividing and trigger cancer cells to die.
Uncontrolled cell division is a hallmark of cancer, which is why many cancer treatments aim to stop cells from multiplying. Now, molecular and computational biology experts from the Biotechnology Center (BIOTEC) of TU Dresden and the Institute of Cancer Research (ICR) in London aim to add new tools to the oncology toolbox. In a new publication in the Journal of Medicinal Chemistry, they present a newly developed set of small molecule inhibitors that stop cancer cells from dividing.
The team, led by Prof. María Teresa Pisabarro and Dr. Jörg Mansfeld, developed small molecules that get in the way of how cancer cells multiply. Their target: a protein complex essential for proper cell division.
“We chose the Anaphase Promoting Complex or APC/C as our target because it is the master regulator of cell division,” explains Dr. Mansfeld, a research group leader formerly at the BIOTEC and now at the ICR, who led the experimental work. “This enzyme acts as a E3 ubiquitin ligase. It labels proteins for destruction. This destruction of key proteins is essential for a cell to progress through the division cycle and multiply.”
The APC/C has been widely studied and identified as a promising cancer therapy target, especially in cancers with KRAS mutation. However, designing a drug that targets the APC/C has long been considered nearly impossible.
“The APC/C is a massive, intricate molecular complex made up of more than 15 different proteins,” says Prof. Pisabarro, a research group leader at the BIOTEC who led the molecule design. “Designing a small molecule to bring down such a huge complex system was like crafting a high-precision mini molecular probe to jam the gears of a massive machine. We are proud to say we succeeded.”
Rational Design, Iterative Testing
The team focused on naturally occurring inhibitors of APC/C and other RING E3 ubiquitin ligases. Using detailed 3D structural data, they reverse-engineered and optimized how these natural molecules interact with their targets – down to the atomic level.
“We identified key molecular features that allow these E3 ubiquitin ligases to bind their partners,” explains Dr. Gloria Ruiz-Gómez, a computational chemist in the Pisabarro group. who was in charge of designing the molecules. “We then designed small molecules with these key features in order to mimic those interactions in a more stable and effective way.”
Designing the new molecules was a collaborative and iterative process. The computational biologists from the Pisabarro group created the initial designs, the Mansfeld group tested them in the lab, and the designs were optimized based on the results. This iterative cycle continued until the team obtained a set of molecules that were effectively blocking the APC/C activity.
Toward Future Cancer Therapies
“RING E3 ubiquitin ligases are the largest family of ubiquitin ligases and play a crucial role in regulating cell cycle,” says Dr. Alena Uvizl, a former Mansfeld Lab researcher who was responsible for the experimental part of the work. “Because all RING E3 ubiquitin ligases share a similar mode of action, our blocking strategy could be extended to other enzymes in this family.”
The team demonstrated that their molecules not only effective block the APC/C activity in vitro but can also stop cells from dividing and trigger cell death in specific human cancer cell lines.
“Our next goal is to improve how these molecules are delivered into living cells. This is a critical step towards exploring therapeutic applications,” says Prof. Pisabarro.
Original Publication:
Gloria Ruiz-Gómez, Alena Uvizl, Gábor Bakos, Jacky K. Leung, M. Teresa Pisabarro, and Jörg Mansfeld: De Novo-Designed APC/C Inhibitors Provide a Rationale for Targeting RING-Type E3 Ubiquitin Ligases. Journal of Medicinal Chemistry (Mai 2025)
Link: https://doi.org/10.1021/acs.jmedchem.5c00416
