Jan 08, 2018
The bright side of an infectious protein: Stress sensors promote yeast cell survival
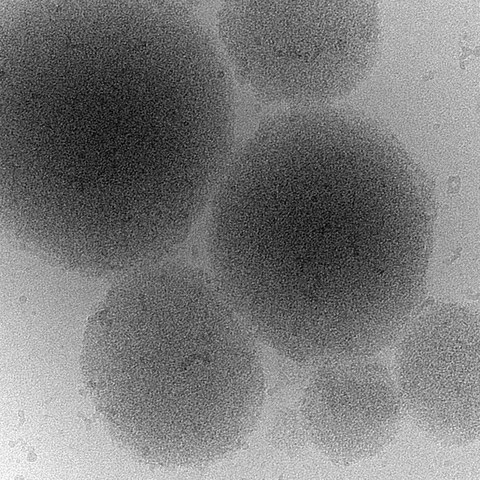
Cryo-Electron Microscopy Image of a biomolecular condensate of a Prion Protein
Prions are self-propagating protein aggregates that can be transmitted between cells. The aggregates are associated with human diseases. Indeed, pathological prions cause mad cow disease and in humans Creutzfeldt-Jakob disease. The aggregation of prion-like proteins is also associated with neurodegeneration as in ALS. The regions within prion-like proteins that are responsible for their aggregation were termed prion-like domains. Despite the important role of prion-like domains in human diseases, a physiological function has remained enigmatic. Researchers at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG), the Biotechnology Center of the TU Dresden (BIOTEC, Grill group), and the Washington University in St. Louis, USA have now identified for the first time a benign, albeit biologically relevant function of prion domains as protein specific stress sensors that allow cells to adapt to and survive environmental stresses. Uncovering the physiological function is an essential first step towards closing a gap in understanding the biological role of prion domains and their transformation into a pathological disease-causing state. The discoveries were published in Science.
Picture: Cryo-Electron Microscopy Image of a biomolecular condensate of a Prion Protein.
Contact:Prof. Dr. Stephan Grill
