Latest news
10 years NCT/UCC
The NCT/UCC Dresden looks back on ten years of cutting-edge research in which modern cancer medicine, innovative technologies and patient-oriented care have been closely interlinked. To mark the anniversary, Minister President Michael Kretschmer and numerous representatives from politics, science and the partner institutions involved visited the site and paid tribute to its outstanding role as a beacon of cancer medicine on the 15th of december. The visit underlined the national importance of the center and the success of the Dresden model of transferring research results quickly and effectively into patient care.
BioBank Dresden was also represented with its own information stand and met with great interest from the many visitors to the anniversary event. The central role of biobanks for innovative, patient-oriented cancer research became clear in many intensive and stimulating discussions - including with the Minister President, representatives of patient advocacy groups, BMFTR employees, lawyers, researchers and doctors. The lively exchange impressively demonstrated the importance of close dialogue between research, clinics, politics and society in order to further strengthen the conditions for future progress in personalized medicine.
----------------------------------------------------------------------------------------------------------------------------------
Visit to Lifelines Biobank and LvL on October 13
On October 13, we had a visit from our partners Lifelines Biobank (NL) and LvL.
Hilde Laeremans and Rick Nijboer (Lifelines Biobank) as well as Waldemar Janzen and Nick Wolter (LvL) were with us.
Together, we took a closer look at the BBD, focusing in particular on our scanner technology and the integrated scanner solutions in the PBMC system. Exciting discussions focused on troubleshooting, robotic systems and the exchange of experiences and solutions. Our guests brought a number of technical challenges with them, and we were able to share our ideas and approaches.
Finally, there was a cozy evening event - a nice end to a day full of exciting impressions and discussions.
We would like to thank you for your visit and look forward to the next meeting!
----------------------------------------------------------------------------------------------------------------------------------
BioBank Dresden at the 13th National Biobank Symposium: Setting the course for a national biobank
From September 22 to 23, 2025, more than 200 experts met at the 13th National Biobank Symposium to discuss the future of biobanking in Germany. The focus was on the concept for a national biobank envisaged in the coalition agreement, which is to serve as a central digital platform to provide many millions of tissue and liquid samples as well as data for medical research.
As part of the Network of University Medicine (NUM), BioBank Dresden is supporting this pioneering project, which was jointly developed by a broad alliance of academic research and industry. The aim is to permanently secure access to quality-assured samples and to further improve the linking of biosamples with health data - especially in the course of digitalization and with a view to the European Health Data Space.
We see the National Biobank as a great opportunity to strengthen Germany as a research location and take biomedical research to a new level.
You can find more information here.
---------------------------------------------------------------------------------------------------------------------------------
Visit from Sweden: Teresa Mortera Blanco visits Biobank Dresden
We are delighted to have recently welcomed Dr. Teresa Mortera Blanco from the renowned Karolinska University in Stockholm to Biobank Dresden. Dr. Mortera Blanco heads the research department of the MDS working group of Prof. Eva Hellström-Lindberg - one of the leading international experts on myelodysplastic syndromes (MDS) and a member of the Nobel Prize Committee for Medicine. She is also responsible for the Karolinska Institute's hematology biobank, which has come into the international spotlight in recent months after a technical failure necessitated a comprehensive restructuring.
In an exciting lecture, Dr. Mortera Blanco presented the future concept of the new biobank, which includes measures to safeguard and avoid technical failures. In her search for innovative solutions, she came across our publication on automated PBMC processing using robotics - a focus of our work at Biobank Dresden. The technologies presented aroused great interest, and so the desire arose to get an idea of our infrastructure and processes on site.
Dr. Mortera Blanco was accompanied by representatives from Hamilton, who shared technical insights and details about our automation solutions during the tour. An inspiring exchange about common challenges, solutions and possible future forms of cooperation between the Dresden and Stockholm sites ensued.
We look back on an insightful day with valuable scientific dialogue and look forward to further exchanges with Dr. Mortera Blanco and the team at the Karolinska Institute.
---------------------------------------------------------------------------------------------------------------------------------
Our close cooperation partner GBN presents itself with a new name and logo.
All important information here
Kickoff of PEDNET-LC
Together with the Freeze Biobank we coordinate the Bioanking in Pednet-LC network. Biosamples are processed and stored according to common standards in either Freiburg or Dresden according to organized center assignments. They are linked to LC registry data and made available to Pednet-LC research.
It is a great pleasure and honor for us as BioBank Dresden to be part of this important project. The funding offers a unique opportunity to work together with partners from all over Germany to improve care and research on Long COVID and similar diseases in children and adolescents. We are proud to play our part in developing tailored, sustainable solutions for affected young people and advancing scientific collaboration in this important area.
---------------------------------------------------------------------------------------------------------------------------------
German Biobank Node becomes part of the University Medicine Network - we at BioBank Dresden are also involved
The German Biobank Node (GBN), which has been driving forward the networking and standardization of biobanks in Germany for years, will be integrated into the Network University Medicine (NUM) on 1 July 2025. This will further strengthen cooperation between biobanks and university research. In future, the GBN will operate under the new name German Biobank Network, which will also incorporate the German Biobank Alliance (GBA).
As part of the GBA and a member of the GBN, BioBank Dresden is also directly affected by this development. In addition, we are currently actively involved in the NUM study NAPKON NUKLEUS, which further underlines our close ties to the university medical research landscape.
Integration into the NUM will create new opportunities for collaboration - for example through even closer links with the data integration centers - and simplify access to quality-assured biosamples and associated data for researchers throughout Germany. This is an important step towards sustainable and future-oriented structures in biomedical research.
We look forward to taking this path together with our partners in the German Biobank Network and the NUM.
The complete press release can be found here.
----------------------------------------------------------------------------------------------------------------------------------
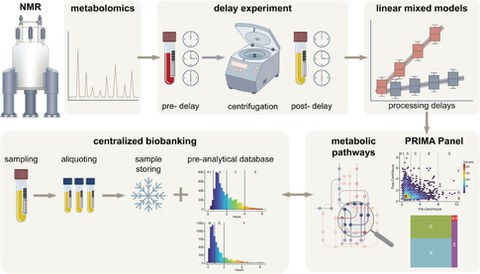
Exciting news from BioBank Dresden (BBD): Our first own publication has been published!
In the study entitled "Validating Centralized Biobanking Workflows for NMR Metabolomics Using the PRIMA Panel", our researchers validate centralized biobanking workflows for NMR metabolomics using the PRIMA panel. This work represents a significant advance in the standardization and quality assurance of biobanking processes and underlines BBD's commitment to research excellence. The full study can be found here.
----------------------------------------------------------------------------------------------------------------------------------
We are pleased to report on the successful 3rd meeting of our Scientific Advisory Board (SAB), which has been held regularly since 2019. This time, the focus was on the exchange with Dr. med. Michael Kiehntopf (3rd from left) , Director of the Institute for Clinical Chemistry and Laboratory Medicine in Jena and the integrated biobank. His concept has many parallels to our approach, which led to an inspiring and insightful dialog. Mr. Kiehntopf is a leading expert in biospecimen quality management, with numerous publications and active membership in international committees and biobank consortia such as ESBB and BBMRI.
In addition, Prof. Dr. Thomas Illig (5th from left), Head of the HUB Hannover and central stakeholder in networks such as NUM, and Prof. Dr. Roland Jahns (4th from left), Head of the IDBW Würzburg and Chairman of the Würzburg Ethics Committee, enriched the discussions with their extensive knowledge and experience.
The exchange was characterized by constructive suggestions for improvement and inspiring perspectives that further strengthen and advance our concept.
We would like to thank all participants for their commitment and look forward to the next steps!
----------------------------------------------------------------------------------------------------------------------------------
In the sixth video entitled "Focus on accreditation - quality management in biobanks", Dr. Heidi Altmann, PD Dr. Dr. Michael Kiehntopf and Prof. Dr. Thomas Illig discuss the GBN quality program, in particular the "Friendly Audits" and the EQA schemes. Michael Kiehntopf begins by emphasizing: "Our aim is to ensure that all samples in the network are of consistent and standardized quality." Heidi Altmann explains the role of the Friendly Audits, which help the biobanks of the German Biobank Alliance (GBA) with accreditation in accordance with DIN EN ISO 20387: "The auditors support our quality management in a very constructive way." Thomas Illig, Head of the recently accredited Hannover Unified Biobank (HUB), summarizes: "We couldn't have done it without GBN."
----------------------------------------------------------------------------------------------------------------------------------
The German Biobank Node (GBN), BBMRI.at and the Swiss Biobanking Platform (SBP), all three "National Nodes" of the European biobanking organization BBMRI-ERIC, have jointly published a new film. It highlights the important role of professional biobanks for research and the promotion of new discoveries in medicine.
With the slogan "Professional biobanks can make your life easier", the two-minute animated film is aimed at researchers and encourages them to work with professional biobanks. The biobanks in the GBN, BBMRI.at and SBP networks offer comprehensive advice and support, work according to international standards to ensure high sample quality, adhere to strict safety protocols and are committed to the long-term success of research.
The resulting film not only serves to provide information, but is also an expression of the intensive cooperation between the "National Nodes" - in line with the founding idea of BBMRI-ERIC. Likewise, as the film shows, collaboration between researchers and biobanks can accelerate research and pave the way to a healthier future.
----------------------------------------------------------------------------------------------------------------------------------
Change in the management of CCP-Bio: Dr. Heidi Altmann and Dr. Katja Steiger take over
In the second quarter of 2024, a significant change in leadership took place within the DKTK Clinical Communication Platform (CCP): Dr. Heidi Altmann and Dr. Katja Steiger have taken over the management of CCP-Bio, the area for networked biobanking, from Prof. Michael Hummel. This handover marks a new phase for the platform, which focuses on optimizing access to biosamples. You can find the complete article here.
----------------------------------------------------------------------------------------------------------------------------------
As part of its anniversary year 2024, the German Biobank Node (GBN) presents a special video series. This series offers insights into the GBN's past successes and activities. As one of the representatives, our spokesperson Dr. Heidi Altmann also has her say.
----------------------------------------------------------------------------------------------------------------------------------
New project launched:
In a delightful partnership with a pioneering environmentally conscious project, we are pleased to announce that LVL has now officially started production of its fully recycled rack series.
----------------------------------------------------------------------------------------------------------------------------------
We had two significant site visits in March:
The esteemed team around Dr. Nadine Volk from NCT Cell and Liquid Biobank // Heidelberg took the opportunity to evaluate our biobank infrastructure with regard to the automated workflow of PBMC isolation (isolation of cells from blood and bone marrow). The overall goal is to provide a homogeneous sample collection for national cancer research across different sites. To this end, the PBMC biobanking process (automated isolation, freezing and storage) was implemented in parallel to the process in Heidelberg. The visit enabled a fruitful exchange and intensive discussions about possible solutions to challenges that arise and potential opportunities for cooperation.
This was followed by a visit from the team led by Dr. Diana Debreceni, Head of the Biobank Facility at the Center for Molecular Fingerprinting at the University of Szeged in Hungary, and Dr. Frank Fleischmann from the Max Planck Institute of Quantum Optics in Garching. The focus was on a tour of our infrastructure, accompanied by lively discussions about the biobanking workflow with a particular focus on automated storage. The background to this is the goal of establishing a national biobanking infrastructure in Hungary for the epidemiological biobanking of healthy samples. The visit was characterized by a lively exchange on topics such as process planning, system configuration, commissioning, validations, troubleshooting and data management in the biobanking context.
At the 11th National Biobanking Symposium in May last year, LVL Technologies GmbH & Co. KG caused a stir by raffling off a custom-made bench with the striking "Biobank" lettering, made with LVL Tubes. This bench, handmade by a carpenter, quickly found a lucky winner in our Quality and Project Manager, Dr. Susann Witt.
After an exciting wait, the bench was placed in our nitrogen storage facility on 20.03.2024 with due ceremony. Mr. Tobias Häßner from LVL Technologies was personally present to bring the bench to its new location and symbolically hand it over to BioBank Dresden. This gesture underlines not only the importance of cooperation between industry and research institutions, but also the innovative strength and sense of community that characterizes the biobank community.
----------------------------------------------------------------------------------------------------------------------------------
Press Release
Ten years of the German Biobank Node (GBN) - A decade of progress in biomedical research
Berlin, 17.01.2024. Human biospecimens stored in biobanks and linked data help to detect diseases at an early stage and treat them successfully. The German Biobank Node (GBN), an umbrella organization for academic biobanks in Germany, was founded to make these valuable resources more easily accessible for national and international research while ensuring the highest quality standards. This year, the GBN is celebrating its tenth anniversary.
Dr. Heidi Altmann comments: "The future of quality-oriented biobanking lies in strong cooperation at national and European level. The work of the GBN helps to accelerate research and develop innovative therapies."
You can read the full press release here. A short article was also published in the current Ärztezeitung.
----------------------------------------------------------------------------------------------------------------------------------
A starter kit is now available online via GBA, which can be used as a tool for setting up biobanks. This was co-developed by our BBD spokesperson Dr. Heid Altmann.
----------------------------------------------------------------------------------------------------------------------------------
Employees of different biobank sites of the German Biobank Alliance (GBA ) have the opportunity to audit each other once a year in the form of so-called "Friendly Audits". These audits support quality management by ensuring that the organization's processes, requirements and guidelines meet the prescribed standards. On 18.12.2023, we successfully conducted and completed our first GBA friendly audit.
----------------------------------------------------------------------------------------------------------------------------------
Dr. Heidi Altmann was elected to the Steering Committee of the German Biobank Alliance for 2024-2025. More details here.
----------------------------------------------------------------------------------------------------------------------------------
Our quality manager Dr. Susann Witt has successfully completed her training and is now an official auditor for German Biobank Node.
----------------------------------------------------------------------------------------------------------------------------------
3Sat showed great insights into the work of our biobank in an exciting documentary on"Collecting, storing and preserving" on 16.12.2023.
The documentary is still available to watch in the media library.
----------------------------------------------------------------------------------------------------------------------------------
From August 21, 2023 to August 2024, you will find the exhibition "From Shadow to Light" in House 136 of the NCT Dresden.
In this context, Julia Schmelzer is showing, among other things, a film project that was created in collaboration with BioBank Dresden and the NCT/UCC and deals with modern cancer medicine and research.
The camera takes the viewer through parts of our DILB, the sober reality of examination equipment and laboratories, while at the same time delving deep into the microworld of microbiological and organic details.