Improvements in clinical cellular therapies
PD Dr. med. Malte von Bonin
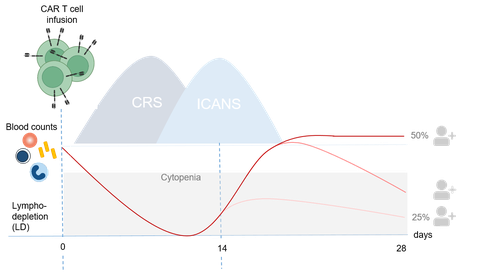
Cellular therapies have evolved within the context of hematopoietic cell transplantation (HSCT). In parallel, adoptive immunotherapies outside of HSCT have also proven the capability of cell based immunotherapies to kill tumors. This particularly gained momentum with advances in genetic engineering. Chimeric antigen receptor modified T lymphocytes (CAR T) targeting CD19 were the first living, genetically modified drugs that received approval in 2017. However, besides effectiveness, tolerability will be the key to clinical success. Our group investigates how to manage pleiotropic systemic inflammatory effects of immunotherapies and thus to improve cancer care.
Our lab focuses on anti-neoplastic immune effector cell (IEC) therapies including CAR T cells and macrophages. This encompasses clinical and laboratory elements related to IEC application. Building on our findings on the disturbance of the bone marrow microenvironment with emphasis on mesenchymal stromal cells (MSCs) in steroid-refractory graft versus host disease (GvHD) , we are particularly interested in changes within the bone marrow stroma which might contribute to IEC-associated protracted hematotoxicity.
- Deciphering immune effector cell associated hematotoxicity
- Understanding the clinical relevance of macrophages as new cell therapeutic agents for cancer treatment in cooperation with Sieweke Lab
Lab members
 © Thomas Albrecht
© Thomas Albrecht
PD Dr. med. Malte von Bonin
|
POSITIONS AND EDUCATION |
|
|---|---|
| since 2023 | CAMINO Group leader Improvements in clinical cellular immunotherapies |
| 2017 - present |
Senior physician, Medical Clinic 1, University hospital Dresden Focus: stem cell transplantation, CAR-T cell therapy, flow cytometry |
| 2013 - 2020 |
DKTK Junior Research Group leader Cancer immunotherapy, German Cancer Consortium (DKTK), Partner site Dresden |
| 2018 |
Habilitation, Technische Universität Dresden, Preclinical characterization of a bispecific antibody directed against CD33 for the therapy of acute myeloid leukemia. |
| 2011 - 2017 |
Consultant, Medical Clinic 1, University hospital Dresden |
| 2009 - 2011 |
GEROK research position, SFB 655 Regeneration in Hematopoiesis (Dr. C. Waskow), Center for Regenerative Therapies Dresden (CRTD) |
| 2004 – 2011 |
Registrar University hospital Dresden |
| 2006 |
Medical doctorate Technische Universität Dresden, In-vivo und in-vitro Pharmakokinetik von Imatinib Mesylat |
| 1997 - 2004 | Medical studies Ernst Moritz-Arndt-Universität Greifswald and TUD |
| CLINICAL TRAINING | |
|---|---|
| 2014 | Board certification Hematology/Oncology |
| 2011 | Board certification Internal Medicine |
| HONORS AND GRANTS | |
|---|---|
| 2022-present | Chameleon CHarActerization of MyELoma bonE marrOw Niche , Co-Principal Investigator |
| 2021-present | MinimaL Machine-Learning-basierte Algorithmen zur Detektion von Resterkrankung bei Patient*innen mit akuter myeloischer Leukämie , Principal Investigator |
| 2013 -2020 |
DKTK Junior research position |
| 2009-2011 | GEROK research position |
MEMBERSHIPS
Deutsche Gesellschaft für Hämatologie und Onkologie (DGHO)
LINKS
 © Thomas Albrecht
© Thomas Albrecht
MD student
NameVivian Arlt
Eine verschlüsselte E-Mail über das SecureMail-Portal versenden (nur für TUD-externe Personen).
 © Thomas Albrecht
© Thomas Albrecht
Technical Assistance
NameClaudia Richter
 © Thomas Albrecht
© Thomas Albrecht
Clinician Scientist
NameDr. med. Maximilian Alexander Röhnert
 © Thomas Albrecht
© Thomas Albrecht
Clinician Scientist
NameJonas Schadt
 © Thomas Albrecht
© Thomas Albrecht
Clinician Scientist
NameDr. med. Raphael Teipel
Selected publications
Krüger T, Wehner R, Herbig M, Krater M, Kramer M, Middeke JM, …, Bornhäuser M, von Bonin, M. Perturbations of mesenchymal stromal cells after allogeneic hematopoietic cell transplantation predispose for bone marrow graft-versus-host-disease. Front Immunol. 2022;13:1005554
Röhnert MA, Kramer M, Schadt J, Ensel P, Thiede C, Krause SW, …, Oelschlagel, U., von Bonin, M. Reproducible measurable residual disease detection by multiparametric flow cytometry in acute myeloid leukemia. Leukemia. 2022;36(9):2208-17
Krüger T, Middeke JM, Stölzel F, Mütherig A, List C, Brandt K, Heidrich K, Teipel R, Ordemann R, Schuler U, Oelschlägel U, Wermke M, Kräter M, Herbig M, Wehner R, Schmitz M, Bornhäuser M, von Bonin M. Reliable isolation of human mesenchymal stromal cells from bone marrow biopsy specimens in patients after allogeneic hematopoietic cell transplantation. Cytotherapy. 2020 Jan;22(1):21-26
We established a profound method to measure bone marrow MSCs in patients for the first time. This method has been used to understand and quantify niche changes.
von Dalowski F, Kramer M, Wermke M, Wehner R, Röllig C, Alakel N, Stölzel F, Parmentier S, Sockel K, Krech M, Schmitz M, Platzbecker U, Schetelig J, Bornhäuser M, von Bonin M. Mesenchymal Stromal Cells for Treatment of Acute Steroid-Refractory GvHD: Clinical Responses and Long-Term Outcome. Stem Cells. 2016 Feb;34(2):357-66.
This study investigates one of the biggest patient cohorts at that time point with steroid refractory GvHD receiving MSCs.
Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, … Bennett, C.,Dazzi, F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416)
Establishment of novel biomarkers to predict MSC response within the context of GvHD.
von Bonin M, Wermke M, Cosgun KN, Thiede C, Bornhauser M, Wagemaker G, Waskow C. In vivo expansion of co-transplanted T cells impacts on tumor re-initiating activity of human acute myeloid leukemia in NSG mice. PLoS One. 2013 Apr 9;8(4):e60680.
Contact
 © Thomas Albrecht
© Thomas Albrecht
Bonin Lab