Clinical Electrophysiology of the Heart
My group focuses on the electrical activity of cardiomyocytes in heart disease. Innate or acquired electrical alterations lead to cardiac arrhythmia and are responsible for a large number of sudden deaths. Consequently, understanding cellular cardiac electrophysiology and pharmacology is of high medical importance.
My areas of interest include electrical and structural cardiac remodeling in hypertrophy and heart failure as well as regulation and pharmacology of cardiac ion channels.
Projects
Electrical cardiac remodeling in hypertrophy and heart failure
Cardiac hypertrophy results in prolongation of the ventricular action potential duration and the emergence of afterdepolarizations. Moreover, it disturbs the order of repolarisation and thereby additionally contributes to the genesis of an arrhythmic substrate. On the level of ion currents, electrical remodelling is characterised by a reduction in repolarising K+ currents and an increase in depolarising currents such as the L-type Ca2+ current and the late Na+ current. We measure ion currents in single cardiomyocyte using patch-clamp technique (Fig. 1, 2).
In addition to change in the plasma membrane of cardiomyocytes, disturbances of cellular Ca2+ homeostasis such as diastolic Ca2+ release, also contribute to the generation of cardiac arrhythmias. We examine cellular Ca2+ homeostasis using Ca2+- dependent changes in the spectra of specific dyes to measure systolic Ca2+ transients as well as diastolic Ca2+ release events.
Fig. 1: Isolated cardiomyocyte undergoing patch-clamp measurements
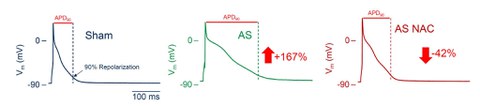
Fig. 2: Representative action potentials of cardiomyoyctes isolated from hearts of a sham-operated rat, a rat after ascending aortic stenosis and a rat after ascending aortic stenosis treated with the reducing agent N-acetylcysteine. The numbers indicate the mean change in the action potential duration.
Literature:
Foltz, W. U.*, Wagner, M.*, Rudakova, E., Volk, T. (2012). N-acetylcysteine prevents electrical remodeling and attenuates cellular hypertrophy in epicardial myocytes of rats with ascending aortic stenosis. Basic Res Cardiol 107, 290.
Volk, T., Noble, P. J., Wagner, M., Noble, D., Ehmke, H. (2005). Ascending aortic stenosis selectively increases action potential-induced Ca2+ influx in epicardial myocytes of the rat left ventricle. Exp Physiol 90, 111-121.
Phosphodiesterase 2 as an antiarrhythmic target
The electrophysiological effects of phosphodiesterase 2 (PDE2), an important enzyme in β-adrenergic signaling, is another focus of my group. PDE2 hydrolyses the second messenger cAMP upon stimulated by another second messenger, cGMP. The increase in PDE2 found in human heart disease is a potential beneficial mechanism, protecting the cardiomyocytes from sustained β-adrenergic stress. Overexpression of PDE2 blunts arrhythmias in an animal model (Fig. 3). We aim to elucidate the cellular mechanisms to translate PDE2 modulation to the clinics as a potential new antiarrhythmic principle.
Fig. 3: A, stimulation of the L-type Ca2+ current by isoprenaline in a wildtype cardiomyocyte (upper panel) and a myocyte overexpressing PDE2A3 (lower panel). B, PDE2A3 overexpression blunts premature ventricular complexes as well as ventricular tachycardia after isoprenaline challenge.
Literature:
Mehel, H., Emons, J., Vettel, C., Wittkopper, K., Seppelt, D., Dewenter, M., Lutz, S., Sossalla, S., Maier, L.S., Lechene, P., Leroy, J., Lefebvre, F., Varin, A., Eschenhagen, T., Nattel, S., Dobrev, D., Zimmermann, W.H., Nikolaev, V.O., Vandecasteele, G., Fischmeister, R., El-Armouche, A. (2013). Phosphodiesterase-2 is up-regulated in human failing hearts and blunts beta-adrenergic responses in cardiomyocytes. J Am CollCardiol 62, 1596-1606.
Regulation of cardiac ion channels by steroids
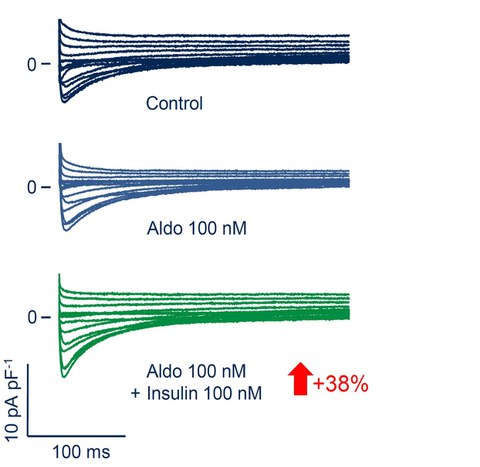
The RALES study underlined the critical role of the mineralocorticoid receptor (MR) in the pathophysiology of heart failure. The actual agonist of the cardiac MR remains elusive. However, since the cardiac MR should constitutively be occupied and potentially activated by glucocorticoids, making the role of the mineralocorticoid aldosterone uncertain. We were able to show that glucocorticoids as well as aldosterone increase the cardiac L-type Ca2+ current (ICaL) by MR activation. Moreover, we were able to identify insulin acting on the PI3-kinase as a critical cofactor in the modulation of ICaL via the MR.
Fig. 4: Insulin is a necessary cofactor of aldosterone in increasing ICaL after 24h. The number in the arrow gives the mean effect.
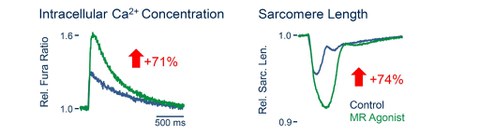
Fig. 5: Increase in intracellular Ca2+ transients (left) and contractility (right) of a cardiomyocyte incubated for 24h with the MR and GR agonist dexamethasone. The numbers in the arrows give the mean effect.
Literature:
Wagner, M., Moritz, A., Volk, T. (2011). Interaction of gonadal steroids and the glucocorticoid corticosterone in the regulation of the L-type Ca2+ current in rat left ventricular cardiomyocytes. Acta Physiol 202, 629-40.
Wagner, M., Rudakova, E., Volk, T. (2008). Aldosterone induced changes in cardiac ion channels are absent in rats on low salt diet and can be prevented by antioxidants in vitro. Pflugers Arch 457, 339-349.
Pharmacology of cardiac ion channels
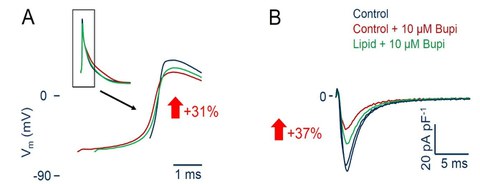
Long acting lipophilic local anesthetics such as bupivacaine are especially prone to cause cardiac depression and arrhythmia when accidently applied to the systemic circulation, creating the clinical need for an antidote. “Lipid rescue“ using conventional lipid emulsions has been proposed as such in a number of case reports. We were able to show on the cellular level that a lipid emulsion can partially reverse the effects of bupivacaine on the cardiac action potential as well as on the fast Na+ current. Reduction of the free bupivacaine concentration by adsorption to the lipid was the most important molecular mechanism of this effect.
Fig. 6: A, bupivacaine reduces the upstroke velocity of the cardiac action potential (red). This can partially be reversed by a lipid emulsion (Lipovenös®) (green). B, likewise, the effect of bupivacaine on the fast cardiac Na+ current (red) can partially be reversed by the lipid emulsion (green). The numbers in the arrows give the mean effect.
Literature:
Wagner, M., Zausig, Y. A., Ruf, S., Rudakova, E., Gruber, M., Graf, B. M., Volk, T. (2014). Lipid rescue reverses the bupivacaine-induced block of the fast Na+ current (INa) in cardiomyocytes of the rat left ventricle. Anesthesiology 120, 724-36.
Wagner, M., Riepe, K. G., Eberhardt, E., Volk, T. (2010). Open channel block of the fast transient outward K+ current by primaquine and chloroquine in rat left ventricular cardiomyocytes. Eur J Pharmacol 647, 13-20.
Methods
Patch-Clamp Technique
Ca2+ Imaging
Echocardiography
ECG Telemetry