Environmental Materials
Table of contents
a) Oxide-based catalytic nanoparticles
Catalytic nanoparticles are synthesized via scalable flame-spray or wet chemical methods. Transition metal oxide catalysts based on manganese-copper-oxide (Hopcalite) are important CO-Oxidation catalysts for filter applications. We have developed new carbon coating methods enhancing the long term stability of these catalysts under humid conditions. Bismuth and zinc oxide base materials are highly efficient absorber materials for the removal of H2S pollutants from air.
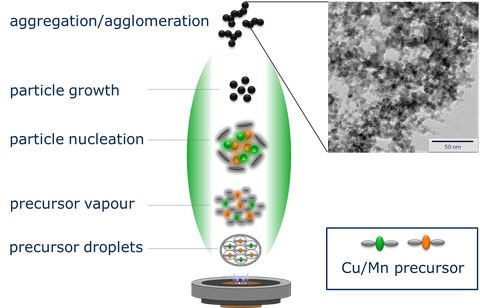
Fig. 1: Flame spray synthesis of oxide catalysts (Hopcalite)
Technologies and applications
- CO-Oxidation catalysts (Hopcalite nanoparticles)
- Formaldehyde oxidation
- Catalytic combustion
- H2S removal
Links
http://www.sciencedirect.com/science/article/pii/S0926337315302423
http://pubs.acs.org/doi/abs/10.1021/acs.iecr.5b01404
http://www.metal-organic-frameworks.eu/filtermedia.shtml
b) Metallic nanoparticles and supported catalysts
Well defined metal nanoparticles supported on porous host materials combine a high catalytic activity and size selectivity due to the pore size engineering. Aerogels provide high porosity and low density.
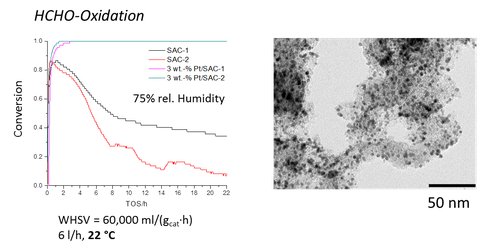
Fig. 2: Metallic nanoparticles supported on spherical activated carbon (SAC-1 and -2) for formaldehyde removal
Technologies and applications
- Water treatment: Catalytic degradation of pharmaceutical residues (Diclofenac)
- Formaldehyde oxidation for indoor applications
- VOC oxidation for indoor applications and cabins
- CO2-electroreduction
c) Porous filter materials
Metal-organic frameworks, porous carbons and polymers are important components for gas filtration. They provide clean air for indoor environments or for personal protection. Tuning the surface functionality is crucial to achieve a high selectivity under working conditions. High humidity applications require in depth knowledge of water adsorption behavior as a competitive adsorption mechanism.

Fig. 3: Porous filter materials: a) MOF Monolith, b) Functional filter textile
Technologies and applications
- Air purification (removal of H2S, NH3, TICs, etc.)
- Greenhouse gas emission control
- CO2-separation, carbon capture technologies
Links
http://onlinelibrary.wiley.com/doi/10.1002/anie.201504572/abstract
http://onlinelibrary.wiley.com/doi/10.1002/ceat.201600232/abstract
