Understanding negative gas adsorption in highly porous networks for the design of pressure amplifying materials
Inhaltsverzeichnis
ERC Advanced Grant: “Amplipore”
ERC Advanced Grant: “Amplipore” ERC Advanced Grant: “Amplipore” ERC Advanced Grant: “Amplipore” ERC Advanced Grant: “Amplipore” ERC Advanced Grant: “Amplipore”
(GA No.: 742743)
Duration: 9/2017-8/2022
Negative gas adsorption (NGA) is a new and counterintuitive phenomenon, for the first time reported in 2016: Normal solid materials with significant outer or inner surface area always take up gas when the pressure in the surrounding reservoir is increased (adsorption). NGA networks instead react at a certain point in the opposite direction: They release gas upon external gas pressure increase, leading to an overall pressure amplification in a closed system (Fig. 1). Comparable phenomena have never been reported before.
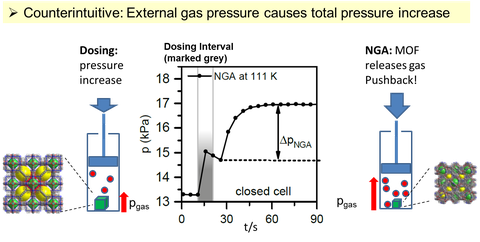
Fig. 1: Counterintuitive NGA: When increasing external gas pressure (left), the MOF releases gas by contraction causing an overall pressure amplification (middle) in the closed system (right).
What is so exciting about NGA? We have a unique material in hand, that counteracts to an external force by force amplification. This phenomenon shows a characteristic negative step in the adsorption isotherm (Fig. 2).
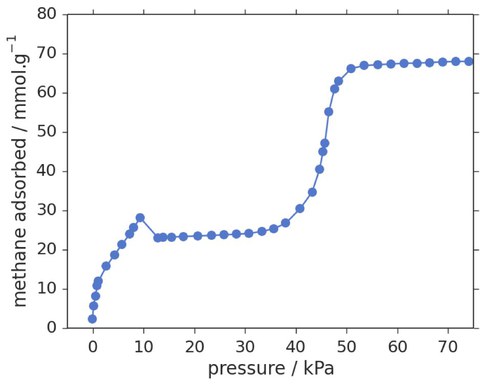
Fig. 2: Methane adsorption isotherm at 111 K (DUT-49).
So far NGA has solely been observed in a handful of coordination polymers, featuring a colossal compression associated with a mesopore-to-micropore transformation (Fig. 3). Gas pressure amplifying materials could lead to important innovations in gas releasing rescue systems, pneumatic control systems (production, transportation), micropumps, microfluidic devices, pneumatic actuators, and artificial lungs.
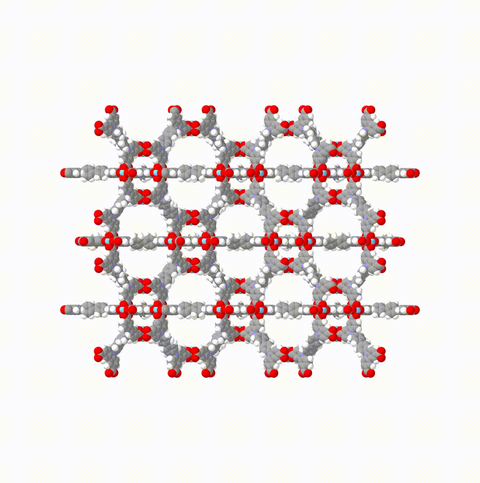
Fig. 3: Structural transformation of DUT-49 (a dynamic MOF changing structure as a response to gas pressure changes).
A fundamental understanding of the physical mechanisms, structures, and thermodynamic boundary conditions is an essential prerequisite for any industrial application of this counterintuitive phenomenon.
List of co-workers:
- Dr. rer. nat. Kartik Maity
- Dr. rer. nat. Ines Hönicke
- Dr. rer. nat. Andreas Schneemann
- Dr. phil. Arpan Hazra
- Dr. rer. nat. Eunji Jin
- Dr. rer. nat. Sebahat Topal
- Dr. rer. nat. Adrian Serpa Gutierrez
- M. Sc. Markus Stötzer
- M. Sc. Bodo Felsner
- M. Sc. Leisan Gilmanova
- M. Sc. Yutong Wang
Former co-workers:
- M. Sc. Fancesco Walenszus
- Dr. rer. nat. Tian Luo
- Dr. rer. nat. Jack. Evans
- M. Sc. Thomas Otto
- M. Sc. Niklas Unglaube
- M. Sc. Nikita Busov
- Dr. rer. nat. Sattwick Haldar
- Dr. rer. nat. Hui-Chun Lee
- Dr. rer. nat. Bikash Garai
- Dr. rer. nat. Steffen Hausdorf
Major achievements:
1. New NGA materials were synthesized and characterized using ligand tuning approach:
- A systematic variation of the ligand length lead to a series of solids, namely DUT-48(Cu), DUT-46(Cu), DUT-50(Cu) and DUT-151(Cu). DUT-50 shows higher pore volume and larger pore size compared to the parent DUT-49 as the second MOF showing NGA transitions closely related to those found in DUT-49 (Fig.4).https://www.nature.com/articles/s41467-019-11565-3
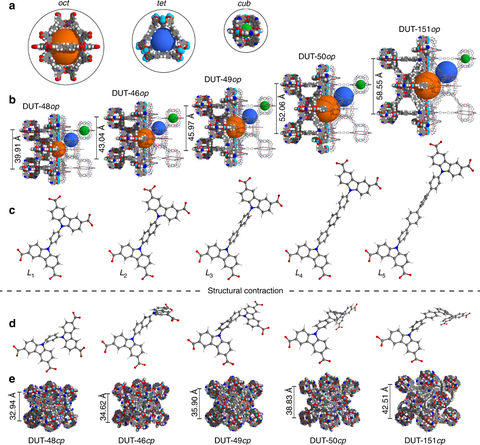
Figure 4. Crystal structures of the MOF series, designed by variation of the ligands length: a) pore system; b) crystal structures of open pore phases; c) ligand molecules in open pore phases; d) ligand molecules in contracted pore phases; e) crystal structures of MOFs in contracted pore phases (Published under CC BY 4.0 license).
- Application of ligand stiffening and softening approached allowed to tune the micromechanics of the resulted frameworks in DUT-147, DUT-148, DUT-160, DUT-162 and DUT-163. This approach allowed to predict the counterintuitive NGA response and pressure amplification behaviour of the framework in adsorption induced structural transformations from stress strain calculations, conducted on the isolated ligand molecules (Fig.5). https://pubs.rsc.org/en/content/articlelanding/2020/SC/D0SC03727C
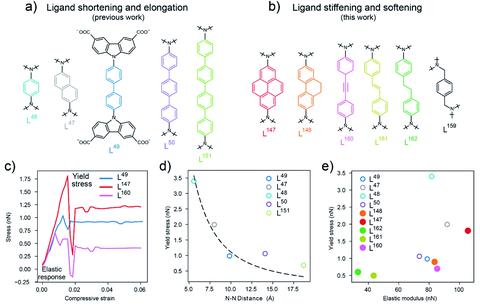
Figure 5. (a) Biscarbazol ligand subunits used in ligand length variation (b) ligand subunits used in the ligand stiffening and softening approach (c) Stress–strain profiles for the ligands in DUT-49 (L49), DUT-147 (L147) and DUT-160 (L160) with the elastic response region and yield stress labelled, (d) correlation of the yield stress (calculated by DFT) and ligand length illustrated by the N–N distance in the ligand backbone. (e) comparison of the yield stress and elastic modulus of the organic ligands (Published under CC BY-NC 4.0 license).
- In order to study the NGA transitions in DUT-49(M) frameworks (M = Co2+, Mn2+, Fe2+, Ni2+, Zn2+, Cd2+) we applied the concept of postsynthetic metal exchange to synthesize a series of DUT-49(M) framework, through single-crystal-to-single-crystal transformation (Fig.6). https://pubs.acs.org/doi/abs/10.1021/acs.chemmater.9b04769
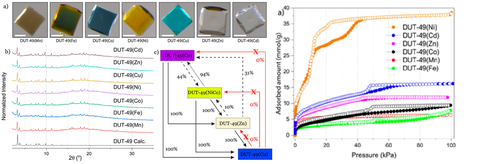
Figure 6. Synthesis of DUT-49(M) frameworks (M = Mn, Fe, Ni, Zn, Co, Cd) using post-synthetic metal functionalization approach: a) single crystals, PXRD patterns and schematic representation of metal exchange; b) nitrogen physisorption experiments on DUT-49(M) at 77K (Adapted from Chem. Mater. 2020, 32, 2, 889–896 with permission of ACS, copyright 2020).
- In the next step, a fluorescent probe was introduced into the DUT-49 framework with the purpose of monitoring NGA by fluorescence and UV-vis spectroscopies. For this reason, the biphenyl moiety in the H4BBCDC ligand of DUT-49 was exchanged by fluorenone, due to the structural similarity and, therefore, expected NGA behaviour (Fig. 7). The adsorption properties of DUT-140(Cu) were investigated and the NGA behaviour was observed upon adsorption of methane at 111 K. To enable the fluorescence detection and to avoid paramagnetic fluorescence quenching, DUT-140(Zn) was synthesized by postsynthetic metal exchange. The influence of the mechanical stress and linker deformation on fluorescence properties was further investigated in desolvation experiments. https://pubs.acs.org/doi/abs/10.1021/acs.chemmater.1c01804
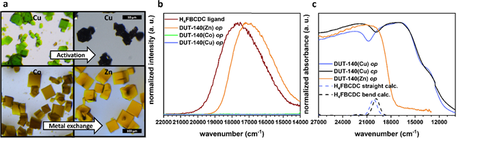
Figure 7. a) Microscopic images of DUT-140(M) crystals, top line: DUT-140(Cu) before and after activation, bottom line: DUT-140(Co) after synthesis and DUT-140(Zn) after metal exchange; b) Fluorescence decay over desolvation from acetone. Spectra are recorded every 5 min for 25 h; c) In situ PXRD over desolvation from EtOH. PXRD was recorded every 115 s over 5.5 h (Adapted from Chem. Mater. 2021, 33, 20, 7964–7971 with permission of ACS, copyright 2021).
2. NGA mechanism was studied in detail using advanced in situ NMR, EPR and X-ray diffraction techniques:
- In situ 129Xe NMR was used to study the influence of host-guest interactions and structural defects on NGA transitions. https://pubs.acs.org/doi/abs/10.1021/acs.chemmater.9b02003
- The impact of artificially engineered defects on NGA in DUT-49(Cu) was explored by in situ 129Xe NMR. https://pubs.acs.org/doi/10.1021/acs.chemmater.0c01059
- The importance of the crystal size on the flexibility and NGA transitions was shown for example in DUT-49(Cu). https://www.nature.com/articles/s41467-018-03979-2
- The role of temperature and adsorbate on NGA in DUT-49(Cu) was investigated using inert gases and hydrocarbons. https://pubs.rsc.org/en/content/articlelanding/2021/fd/d0fd00013b
- The energetics of NGA was studied in details using low-temperature calorimetry. https://pubs.acs.org/doi/abs/10.1021/acs.chemmater.0c00417
- The counterintuitive switching of thermal expansion in the solvated DUT-49(M) frameworks was studied by synchrotron single crystal X-ray diffraction. https://pubs.rsc.org/en/content/articlelanding/2020/ta/d0ta06830f
- The combination of PFG NMR with MD simulations has revealed the distinctive diffusion behavior of DUT-49(Cu) during adsorption and desorption of n-butane at 298 K. https://pubs.acs.org/doi/abs/10.1021/acs.jpclett.0c02745
3. In depth understanding and requirements for NGA was derived from in silico studies:
- Using multiscale computer simulations, the origin of NGA in DUT-49(Cu) was explained by mechanics and thermodynamics. https://www.sciencedirect.com/science/article/pii/S2451929416302315
- The generic thermodynamic conditions for NGA were described. https://pubs.rsc.org/en/content/articlelanding/2019/sc/c9sc01299k
- The Complete Thermodynamic Landscape of Gas Adsorption for DUT-49(Cu) was successfully calculated. https://pubs.acs.org/doi/10.1021/jacs.1c00522
4. We identified conditions for massive pressure amplification in DUT-49(Cu) and DUT-50(Cu) frameworks using carbon dioxide as a stimulus and validated this finding by experimental demonstration of pressure amplification up to 428 kPa.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202100549
Publications:
-
"Chemically Stable Carbazole-Based Imine Covalent Organic Frameworks with Acidochromic Response for Humidity Control Applications", L. Gilmanova, V. Bon, L. Shupletsov, D. Pohl, M. Rauche, E. Brunner, S. Kaskel, J. Amer. Chem. Soc. 2021, 143 (44), 18368–18373.
-
"Integration of Fluorescent Functionality into Pressure-Amplifying Metal–Organic Frameworks", F. Walenszus, J. D. Evans, V. Bon, F. Schwotzer, I. Senkovska, S. Kaskel, Chem. Mater. 2021, 33, 7964−7971.
-
"Unraveling the Guest-Induced Switchability in the Metal-Organic Framework DUT-13 (Zn)", B. Felsner, V. Bon, J. D. Evans, F. Schwotzer, R. Grünker, I. Senkovska, S. Kaskel, Chem. Eur. J. 2021, 37, 9708-9715.
-
"Charting the Complete Thermodynamic Landscape of Gas Adsorption for a Responsive Metal–Organic Framework", R. Goeminne, S. Krause, S. Kaskel, T. Verstraelen, J. D. Evans, J. Am. Chem. Soc. 2021, 143 (11), 4143–4147
-
"Massive pressure amplification by stimulated contraction of mesoporous frameworks", V. Bon, S. Krause, I. Senkovska, N. Grimm, D. Wallacher, D. M. Többens, S. Kaskel, Angew. Chem., Int. Ed. 2021, 60 (11), 735-11739.
- "Elucidating the Structural Evolution of a Highly Porous Responsive Metal–Organic Framework (DUT-49(M)) upon Guest Desorption by Time-Resolved in Situ Powder X-ray Diffraction", B. Garai, V. Bon, F. Walenszus, A. Khadiev, D. V. Novikov, S. Kaskel, Cryst. Growth Des. 2021, 21 (1), 270–276.
-
"Molecular Diffusion in a Flexible Mesoporous Metal–Organic Framework over the Course of Structural Contraction", F. Walenszus, V. Bon, J. D. Evans, S. Kaskel, M. Dvoyashkin, J.Phys.Chem.Lett. 2020, 11, 9696−9701.
- "Structural Transitions of the Metal–Organic Framework DUT-49(Cu) upon Physi- and Chemisorption Studied by in Situ Electron Paramagnetic Resonance Spectroscopy", D. M. Polyukhov, S. Krause, V. Bon, A. S. Poryvaev, S. Kaskel, M. V. Fedin, J. Phys. Chem. Lett. 2020, 11 (15), 5856–5862.
- "Four-dimensional metal-organic frameworks", J. D. Evans, V. Bon, I. Senkovska, H.-C. Lee, S. Kaskel, Nat. Commun. 2020, 11, 2690.
- "Engineering micromechanics of soft porouscrystals for negative gas adsorption", S. Krause, J. D. Evans, V. Bon, I. Senkovska, S. Ehrling, P. Iacomi, D. M. Többens, D. Wallacher, M. S. Weiss, B. Zheng, P. G. Yot, G. Maurin, P. L. Llewellyn, F.-X. Coudert, S. Kaskel, Chem. Sci. 2020, 11, 9468–9479.
-
"The Role of Temperature and Adsorbate on Negative Gas Adsorption in the Mesoporous Metal-Organic Framework DUT-49", S. Krause, J. D. Evans, V. Bon, I. Senkovska, F.-X. Coudert, D. M. Többens, D. Wallacher, N. Grimm, S. Kaskel, Faraday Discuss. 2021, 225, 168-183.
- "Reversible switching between positive and negative thermal expansion in a metal-organic framework DUT-49 J", B. Garai, V. Bon, A. Efimova, M. Gerlach, I. Senkovska, S. Kaskel . Mater. Chem. A 2020, 8, 20420-20428.
-
"Impact of Defects and Crystal Size on Negative Gas Adsorption in DUT-49 Analyzed by In Situ129Xe NMR Spectroscopy", S. Krause, F. S. Reuter, S. Ehrling, V. Bon, I. Senkovska, S. Kaskel, E. Brunner, Chem. Mater. 2020, 32 (11), 4641–4650.
-
"Low Temperature Calorimetry Coupled with Molecular Simulations for an In-Depth Characterization of the Guest-Dependent Compliant Behavior of MOFsP", Iacomi, B. Zheng, S. Krause, S. Kaskel, G. Maurin, P. L. Llewellyn, Chem. Mater. 2020, 32 (8), 3489–3498
-
"Tunable Flexibility and Porosity of the Metal–Organic Framework DUT-49 through Postsynthetic Metal Exchange", B. Garai; V. Bon; S. Krause; F. Schwotzer; M. Gerlach; I. Senkovska; S. Kaskel, Chemistry of Materials 2020, 32 (2), 889-896.
-
"New insights into solvent-induced structural changes of 13C labelled metal–organic frameworks by solid state NMR", M. Rauche; S. Ehrling; S. Krause; I. Senkovska; S. Kaskel; E. Brunner, Chemical Communications 2019, 55 (62), 9140-9143.
-
"High-Pressure in Situ 129Xe NMR Spectroscopy: Insights into Switching Mechanisms of Flexible Metal–Organic Frameworks Isoreticular to DUT-49", F. Kolbe; S. Krause; V. Bon; I. Senkovska; S. Kaskel; E. Brunner, Chemistry of Materials 2019, 31 (16), 6193-6201.
-
"Towards general network architecture design criteria for negative gas adsorption transitions in ultraporous frameworks", S. Krause, J. D. Evans, V. Bon, I. Senkovska, P. Iacomi, F. Kolbe, S. Ehrling, E. Troschke, J. Getzschmann, D. M. Többens, A. Franz, D. Wallacher, P. G. Yot, G. Maurin, E. Brunner, P. L. Llewellyn, F.-X. Coudert, S. Kaskel, Nat. Commun. 2019,10 (1), 3632.
-
“Exploring the thermodynamic criteria for responsive adsorption processes”, J. Evans, S. Krause, S. Kaskel, M. Sweatman and L. Sarkisov, Chemical Science 2019, 10 (19), 5011-5017.
-
“The effect of crystallite size on pressure amplification in switchable porous solids”, S. Krause, V. Bon, I. Senkovska, D. M. Többens, D. Wallacher, R. S. Pillai, G. Maurin and S. Kaskel, Nat. Commun. 2018, 9, 1573.
- “Adsorption Contraction Mechanics: Understanding Breathing Energetics in Isoreticular Metal-Organic Frameworks”, S. Krause, J. Evans, V. Bon, I. Senkovska, S. Ehrling, U Stoeck, P. Yot, P. Iacomi, P. Llewellyn, G. Maurin, F.-X. Coudert and S. Kaskel, J. Phys. Chem. C 2018, 122 (33), 19171–19179.
- “In Situ Monitoring of Unique Switching Transitions in the Pressure-Amplifying Flexible Framework Material DUT-49 by High-Pressure 129Xe NMR Spectroscopy”, J. Schaber, S. Krause, S. Paasch, I. Senkovska, V. Bon, D. M. Többens, D. Wallacher, Stefan Kaskel and E. Brunner, J. Phys. Chem. C, 2017, 121, 5195–5200.
- “Origins of Negative Gas Adsorption”, J. D. Evans, L. Bocquet and F.-X. Coudert, Chem 2016, 1, 873–886.
- “A pressure-amplifying framework material with negative gas adsorption transitions”, S. Krause, V. Bon, I. Senkovska, U Stoeck, D. Wallacher, D. M. Többens, S. Zander, R. S. Pillai, G. Maurin, F.-X. Coudert and S. Kaskel, Nature 2016, 532, 348–352.
