Jun 09, 2023
Nature Synthesis - Redox-neutral conversion of ubiquitous PV-sources, a white phosphorus workaround
A blueprint for a more sustainable phosphorus chemistry
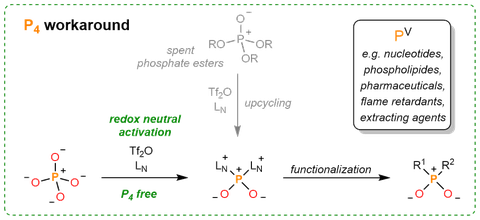
Phosphorus and its compounds are a cornerstone of biological life, as well as finding applications in material science, for example, as flame retardants, battery electrolytes or catalysts. In most applications, phosphorus-containing fine chemicals are in the naturally occurring and most stable oxidation state +V of the element; however, syntheses to these compounds from primary PV sources rely on an energy-consuming and wasteful redox detour via elemental white phosphorus (P4) and its subsequent (oxy)chlorination to PCl3, PCl5 and POCl3.
Now, writing in Nature Synthesis, Jan Weigand and co-workers report an approach that bypasses the energy-intensive and wasteful production of P4 and its (oxy)-chlorinated products as indispensable intermediates. In this work, versatile PO2+ phosphorylation reagents are directly generated from ubiquitous PV sources, thus, providing a redox-neutral access to a range of value-added PV chemicals downstream of low-cost phosphoric acid or other phosphate sources. DOI:10.1038/s44160-023-00344-0.
See also the Nature Synthesis news & views article by Dr. Scott (University of Bath)