3D metal aerogels
Aerogels are macroscopic, highly porous solid bodies, consisting of nanostructured, sponge-like networks whose pores are filled with air. Their high porosity (up to 95% and more), extremely low density (up to 0.0011 g/cm-3) and open porous structure (pore diameters from <1 to over 100 nm) make them a unique class of materials.

Fig.1
Fig. 1: A metal aerogel on different levels: Metal aerogels mostly form black solids (left), which have a sponge-like gel structure (middle-left). The gel itself consists of strands (center-right), which are built up of aggregated nanoparticles. Energy dispersive X-ray spectroscopy coupled with scanning transmission electron microscopy can be used to visualize the elemental distribution in the gel strands (right).
Typically, aerogels are synthesized using a sol-gel-like process. But in the final stage, the network penetrating solvent is gently removed by freeze-drying or supercritical drying to avoid high capillary forces damaging or altering the fragile structure. The transfer of this principle to nanotechnology using colloidal metal nanoparticles as building blocks for the gel formation was first achieved by our group in 2009, establishing a simple way to produce pure metallic aerogels from noble metals (e.g. Au, Ag, Pt and Pd), their respective mixtures (e.g. AuPt, AuAg and PdPt) as well as less-noble metals like Cu, Co and Ni.
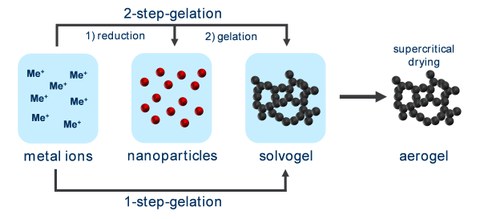
Fig.2
Fig. 2: Scheme for the synthesis of metal aerogels. The synthesis can be divided into the stages of nanoparticle preparation and gelation. Both steps can be performed separately (2-step gelation) or together (1-step gelation). Finally, a special drying technique needs to be applied to obtain aerogels.
To initiate the gelation of the metal nanoparticles mostly a controlled destabilization is required. This can be achieved by changing the polarity of the solvent, adding an electrolyte or by (oxidative) removal of the stabilizing nanoparticle ligands. To this day, a variety of approaches have been developed to realize gelation as simple and as fast as possible. This includes ligand-free synthesis pathways, freeze-gelation or gelation in different solvents, such as H2O and EtOH. Ultimately, disordered, complex, highly porous superstructures with surprisingly high surface areas are formed. Although the gel bodies form monoliths up to several millimeters in size, they still reflect many of the extraordinary properties of the nanomaterials that create them.
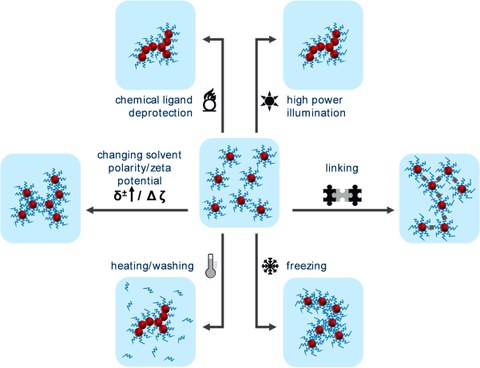
Fig.3
Fig. 3: Different methods for the destabilization of colloidal metal nanoparticles to initiate gelation.
The combination of that open, highly porous and self-supporting gel network with excellent electrical conductivity and high catalytic activity, make metal aerogels ideal candidates as a new class of electrocatalysts. This potential was recognized in our group and confirmed for the first time in the electrooxidation of ethanol (EOR). The use of cyclodextrin-modified Pd aerogels lead to a doubling of the current density in EOR compared to the commercial catalyst (Pd/C). Besides the synthesis and application of bimetallic aerogels, the modification of the morphology of the gel networks also represents a key aspect of our work. For example, the galvanic replacement reaction was used to create porous PdNi and PtNi hollow spheres. These building blocks were subsequently gelated. The use of this gel networks in the oxygen reduction reaction (ORR) led to an 18-fold increase in mass-related activity compared to the standard carbon-supported catalyst. In the course of the energy transition, our work is also focused on the implementation of aerogels in the fuel cell technology. We were able to integrate PtNi aerogels into a 1 cm2 electrode with our cooperation partners from the Paul Scherrer Institute (Switzerland) and test them in a prototype fuel cell. The aerogel catalyst showed a threefold higher catalytic activity than the Pt/C catalyst.
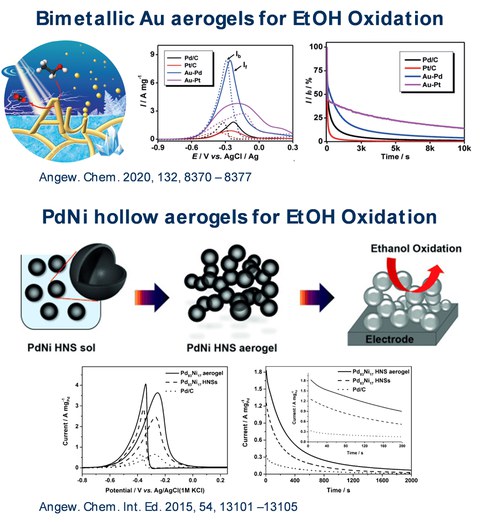
Fig.4
Fig. 4: Selected examples for the application of metal aerogels in the electrocatalytic oxidation of EtOH. The metal aerogels show higher activities and improved long-term stabilities compared to the standard carbon supported catalyst.
In addition to the application in electrocatalysis, a strong point of interest of our group is to investigate the mechanisms of nanoparticle gelation and the influence of individual reactants. In addition to theoretical considerations, we investigate the influence of ionic effects on the gelation, the choice of solvent, the universal role of the reducing agent, and also the mechanical properties of the metal gels.
Currently, our research focuses on aerogel catalysts for the electrochemical reduction of CO2 in order to sustainably obtain new basic chemicals from the greenhouse gas. Furthermore, we deal with the scaling of metal aerogel synthesis and transfer so-called high entropy alloy nanomaterials into gel structures in order to subsequently investigate their electrochemical properties.
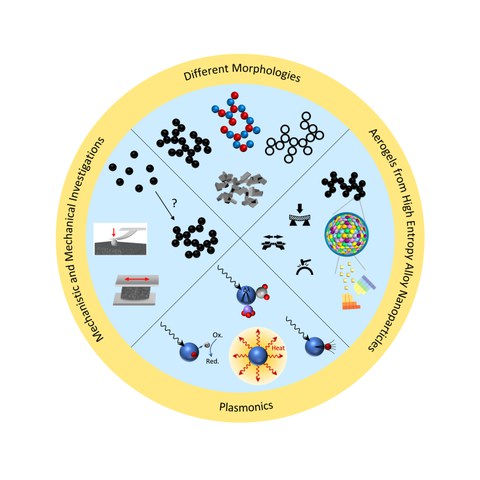
Fig.5
Fig. 5: Further research fields of the Eychmüller group in the field of metal aerogels.
Recommended Literature:
(1) Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A. K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chemie - Int. Ed. 2017, 56 (43), 13200–13221. https://doi.org/10.1002/anie.201611552.
(2) Bigall, N. C.; Herrmann, A. K.; Vogel, M.; Rose, M.; Simon, P.; Carrillo-Cabrera, W.; Dorfs, D.; Kaskel, S.; Gaponik, N.; Eychmüller, A. Hydrogels and Aerogels from Noble Metal Nanoparticles. Angew. Chemie - Int. Ed. 2009, 48 (51), 9731–9734. https://doi.org/10.1002/anie.200902543.
(3) Liu, W.; Herrmann, A. K.; Geiger, D.; Borchardt, L.; Simon, F.; Kaskel, S.; Gaponik, N.; Eychmüller, A. High-Performance Electrocatalysis on Palladium Aerogels. Angew. Chemie - Int. Ed. 2012, 51 (23), 5743–5747. https://doi.org/10.1002/anie.201108575.
(4) Cai, B.; Wen, D.; Liu, W.; Herrmann, A. K.; Benad, A.; Eychmüller, A. Function-Led Design of Aerogels: Self-Assembly of Alloyed PdNi Hollow Nanospheres for Efficient Electrocatalysis. Angew. Chemie - Int. Ed. 2015, 54 (44), 13101–13105. https://doi.org/10.1002/anie.201505307.
(5) Henning, S.; Ishikawa, H.; Kühn, L.; Herranz, J.; Müller, E.; Eychmüller, A.; Schmidt, T. J. Unsupported Pt-Ni Aerogels with Enhanced High Current Performance and Durability in Fuel Cell Cathodes. Angew. Chemie - Int. Ed. 2017, 56 (36), 10707–10710. https://doi.org/10.1002/anie.201704253.
(6) Du, R.; Wang, J.; Wang, Y.; Hübner, R.; Fan, X.; Senkovska, I.; Hu, Y.; Kaskel, S.; Eychmüller, A. Unveiling Reductant Chemistry in Fabricating Noble Metal Aerogels for Superior Oxygen Evolution and Ethanol Oxidation. Nat. Commun. 2020, 11 (1), 1–10. https://doi.org/10.1038/s41467-020-15391-w.
