Synthesis of semiconductor nanomaterials
Colloidal semiconductor nanocrystals (NCs) have evolved during the last three decades from purely fundamental concepts to real commercial products. One of the latest and probably the most fascinating examples of successful commercialization of semiconductor NCs is a line-up of Samsung QLED TVs, in which InP-based NCs are employed as color converters. These nanomaterials benefit on one hand from their unique size-dependent optoelectronic properties, based on quantum confinement effects, arising when the size of semiconductors shrinks down to a few nanometers. On the other hand, their solution-based synthesis is a remarkably simple procedure that can be implemented in nearly any moderately equipped chemical laboratory by using common glassware and available chemicals.
Abb 1. Semiconductor NCs under UV-Lamp.
To date, many approaches have been developed to prepare a wide range of semiconductor NCs, including the II−VI, IV−VI, IB−VI, III−V, VIB−VIA, I−III−VI, I−II−IV-VI, and I−IV−VII families with their typical members CdSe, PbS, Cu2−xS, InP, MoS2, CuInS2, Cu2ZnSnS4, and CsPbBr3, respectively. Altering the conditions of the synthesis makes it possible to tune the size of these particles, their shape, and the crystal structure.
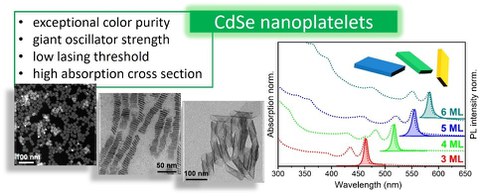
In our group, we develop several types of semiconductor nanomaterials employing either direct synthesis or post-synthetic chemical transformations.1 Among recent examples are the direct synthesis of six monolayer thick CdSe nanoplatelets (NPLs)2 and blue-green emitting compositionally tuneable CdSexS1-x NPLs.
Abb 2. CdSe-based nanoplatelets
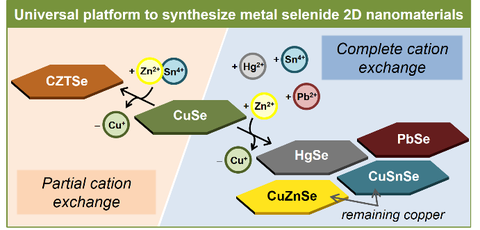
CdSe NPLs can further be exploited as a template in the synthesis of other semiconductor nanomaterials, such as PbSe NPLs3 with varied thickness and CdxHg1-xSe NPLs4-5 which are active in the near infrared (NIR) region. In both cases, we use a cation exchange strategy consisting in a controllable replacement of so called host cations of the template with guest cations (i.e. Pb2+ and Hg2+, respectively). Another appropriate template material is copper chalcogenide NCs, in which copper can easily be replaced by a range of different cations. This exchange can be either complete or partial, leading to the formation of more complex alloy compositions, as we recently demonstrated on the example of CuSe nanosheets transforming them into HgSe, PbSe, CuZnSe, CuSnSe, and Cu-Zn-Sn-Se.6
Abb 3. Cation exchange
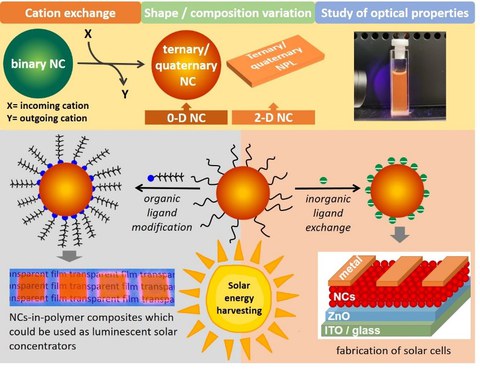
Cd/Pb chalcogenide-based NCs are by far the most studied classes of semiconductor NCs due to their exemplary optical properties. However, their toxicity poses a limit to their widespread application, in particular in biological systems. Therefore, we search for alternatives that can replace Cd/Pb-containing NCs, focusing on I-III-VI semiconductors. Here, direct synthesis of binary IB-VI NCs provides a perfect template with well-controllable shape for subsequent partial cation exchange resulting in ternary and quaternary compositions.7-8 Keeping in view the properties of the synthesized NCs, such as large Stokes shift, high absorption coefficients, and tuneable bandgap, we aim to establish them as active materials for application in solar energy harvesting, particularly in luminescent solar concentrators and solar cells in collaboration with the groups of Dr. F. Paulus and Prof. Y. Vaynzof (Integrated Centre for Applied Physics and Photonic Materials and Centre for Advancing Electronics Dresden). To integrate these NCs in different devices, their surface should be modified by ligand exchange which improves compatibility with polymer matrices and enhances charge transport within NC-solids.
Abb 4. I-III-VI NCs via cation and ligand exchange.
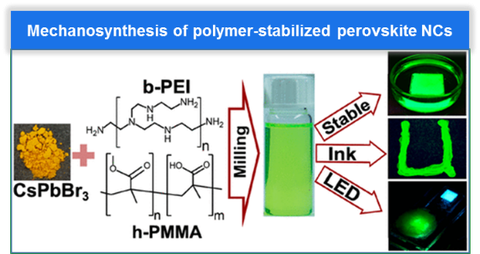
As a newcomer to a family of semiconductor NCs, perovskite NCs exhibit many excellent optoelectronic properties. More importantly, the relatively low formation energy and high defect tolerance of these perovskites enable the synthesis of their highly fluorescent NCs via relatively simple synthetic procedures at lowtemperature. Herein, we developed a synthetic strategy for the preparation of high-performance perovskite NCs based on mechanical ball milling. By introducing specially designed polymer-based ligands in in-situ synthesis, highly stable, highly fluorescent, and easily film-forming perovskite NCs can be easily obtained, which shows a great prospect as a fluorescence conversion medium.9-10
Abb 5. Perovskite NCs in polymers.
Another class of nanomaterials being developed in our group is transition metal dichalcogenides (TMDs). They have properties ranging from insulating through semiconducting to metallic, opening a wide variety of their potential applications from catalysis and energy storage to optoelectronics, spintronics, and valleytronics. In particular, TMDs have a great potential as emerging inexpensive alternatives to noble metal-based catalysts in electrochemical hydrogen evolution. We develop a straightforward, low-cost, and general colloidal synthesis of various 2D TMDs, such as MoS(Se)2 and WS(Se)2, as well as their heterostructures, in the absence of organic ligands. This new preparation route provides many benefits including relatively mild reaction conditions, high reproducibility, high yields, easy upscaling, no post-synthetic treatment steps to enhance their catalytic activity, and, finally, especially for MoS2 nanosheets, high activity in the hydrogen evolution reaction.11
Abb 6. Transition metal dichalcogenides nanoparticles
References
1. Lesnyak, V., Chemical Transformations of Colloidal Semiconductor Nanocrystals Advance Their Applications. J. Phys. Chem. Lett. 2021, 12, 12310-12322.
2. Meerbach, C.; Wu, C.; Erwin, S. C.; Dang, Z.; Prudnikau, A.; Lesnyak, V., Halide-Assisted Synthesis of Cadmium Chalcogenide Nanoplatelets. Chem. Mater. 2020, 32 (1), 566-574.
3. Galle, T.; Samadi Khoshkhoo, M.; Martin-Garcia, B.; Meerbach, C.; Sayevich, V.; Koitzsch, A.; Lesnyak, V.; Eychmüller, A., Colloidal PbSe Nanoplatelets of Varied Thickness with Tunable Optical Properties. Chem. Mater. 2019, 31 (10), 3803-3811.
4. Galle, T.; Kazes, M.; Hübner, R.; Lox, J.; Samadi Khoshkhoo, M.; Sonntag, L.; Tietze, R.; Sayevich, V.; Oron, D.; Koitzsch, A.; Lesnyak, V.; Eychmüller, A., Colloidal Mercury-Doped CdSe Nanoplatelets with Dual Fluorescence. Chem. Mater. 2019, 31 (14), 5065-5074.
5. Mitrofanov, A.; Prudnikau, A.; Di Stasio, F.; Weiß, N.; Hübner, R.; Dominic, A. M.; Borchert, K. B. L.; Lesnyak, V.; Eychmüller, A., Near-Infrared-Emitting CdxHg1–xSe-Based Core/Shell Nanoplatelets. Chem. Mater. 2021, 33 (19), 7693-7702.
6. Shamraienko, V.; Spittel, D.; Hübner, R.; Samadi Khoshkhoo, M.; Weiß, N.; Georgi, M.; Borchert, K. B. L.; Schwarz, D.; Lesnyak, V.; Eychmüller, A., Cation exchange on colloidal copper selenide nanosheets: a route to two-dimensional metal selenide nanomaterials. J. Mater. Chem. C 2021, 9 (46), 16523-16535.
7. Lox, J. F. L.; Dang, Z.; Dzhagan, V. M.; Spittel, D.; Martín-García, B.; Moreels, I.; Zahn, D. R. T.; Lesnyak, V., Near-Infrared Cu–In–Se-Based Colloidal Nanocrystals via Cation Exchange. Chem. Mater. 2018, 30 (8), 2607-2617.
8. Lox, J. F. L.; Dang, Z.; Lê Anh, M.; Hollinger, E.; Lesnyak, V., Colloidal Cu–Zn–In–S-Based Disk-Shaped Nanocookies. Chem. Mater. 2019, 31 (8), 2873-2883.
9. Jiang, G.; Guhrenz, C.; Kirch, A.; Sonntag, L.; Bauer, C.; Fan, X.; Wang, J.; Reineke, S.; Gaponik, N.; Eychmüller, A., Highly Luminescent and Water-Resistant CsPbBr3–CsPb2Br5 Perovskite Nanocrystals Coordinated with Partially Hydrolyzed Poly(methyl methacrylate) and Polyethylenimine. ACS Nano 2019, 13 (9), 10386-10396.
10. Jiang, G.; Erdem, O.; Hübner, R.; Georgi, M.; Wei, W.; Fan, X.; Wang, J.; Demir, H. V.; Gaponik, N., Mechanosynthesis of polymer-stabilized lead bromide perovskites: insight into the formation and phase conversion of nanoparticles. Nano Res. 2021, 14 (4), 1078-1086.
11. Meerbach, C.; Klemmed, B.; Spittel, D.; Bauer, C.; Park, Y. J.; Hübner, R.; Jeong, H. Y.; Erb, D.; Shin, H. S.; Lesnyak, V.; Eychmüller, A., General Colloidal Synthesis of Transition-Metal Disulfide Nanomaterials as Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12 (11), 13148-13155
For continuation see Applications of semiconductor nanomatrials