Organic Semiconductors
What are organic semiconductors?
The bonding of sp2-hybridised carbon
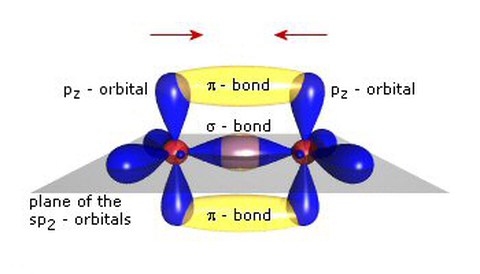
Organic Semiconductors are based on the unusual properties of the carbon atom: Among other configurations, it can form the so-called sp2-hybridisations where the sp2-orbitals form a triangle within a plane and the pz-orbitals are in the plane perpendicular to it. An s-bond between two carbons can then be formed by formation of an orbital overlap of two sp2-orbitals. The energy difference between the occupied binding orbitals and the unoccupied anti-binding orbitals is quite large and well beyond the visible spectral range. Correspondingly, longer chains of bound carbon atoms would have a large gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), leading to insulating properties.
However, in the sp2-hybridisation, the pz-orbitals form additionally p -bonds. These bonds have much smaller energetic difference between the HOMO and LUMO, leading to strong absorption in or near the visible spectral range and to semiconducting properties:
Small-molecule organic semiconductors
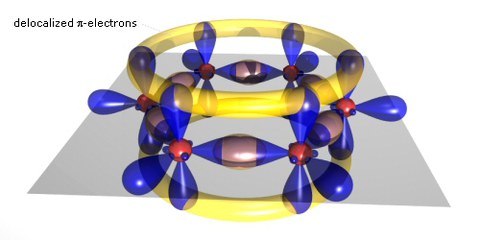
Scheme of a benzene ring
If carbon atoms form larger molecules, typically with benzene rings as the basic unit, the p-bonds become delocalized and form a p-system which often has the extensions of the molecule. The gap between occupied and empty states in these p-systems becomes smaller with increasing delocalization, leading to absorption and fluorescence in the visible. These substances can be prepared as molecular single crystals. Due to the close coupling of the p -systems of the molecules in these crystals, they show in a purified form remarkable transport properties, including band transport up to room temperature with mobilities of 1-10 cm2/Vs. Most of the molecules can also be easily evaporated to form polycrystalline (hopping transport with mobilities typically around 10-3 cm2/Vs at 300K) or amorphous (hopping with mobilities typically around 10-5 cm2/Vs at 300K) layers.
Polymer organic semiconductors
If a long chain of carbon atoms is formed, the p -bonds become delocalized along the chain and form a one-dimensional electronic system.
The 1D-band which results has considerable band width (on the scale of an eV), i.e., we have a 1D semiconductor with a filled valence band originating from the HOMOs and an empty conduction band originating from the LUMOs.
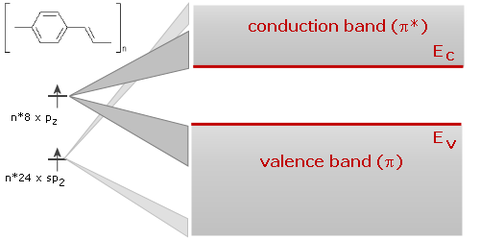
energy structure of polymer organics
The transport properties of such polymers are usually determined by defects in the 1D-chains or by hopping from chain to chain. Therefore, the samples do not show band transport, but thermally activated hopping. Polymer organic semiconductors are usually deposited in wet processes, like spin-coating or printing.