Jun 17, 2025
ERC Advanced Grant: Frank Buchholz (TUD) once again receives the highest endowed EU funding award for precise genome editing

Prof. Frank Buchholz
The prospect of a cure for hereditary and infectious diseases is driving research in the field of genome editing forward at a rapid pace. Numerous ongoing clinical trials and the approval of the first genome editing therapy point to a wide range of treatment options for diseases that were previously considered incurable. Usually, scientists attempt to repair the disease-causing DNA segment in the host cell. Researchers at the Carl Gustav Carus Faculty of Medicine at TU Dresden led by molecular biologist Prof. Frank Buchholz now want to use highly specific editing tools to develop a new class of genome editing technologies that will significantly expand the potential for life-saving therapies. For his DC-PGE project (DNA-binding domain Conditioned Precision Genome Editing), Prof. Frank Buchholz has been awarded an Advanced Grant from the European Research Council (ERC), the highest endowed European funding of almost EUR 2.4 million over a period of five years. Frank Buchholz has thus succeeded in acquiring his second ERC Advanced Grant.
The discovery of the CRISPR-Cas9 gene scissors in 2012 was tantamount to a revolution in science. A comparatively simple procedure made it possible to precisely modify the genetic material of plants, animals and even humans. In 2020, the two discoverers of this method were awarded the Nobel Prize in Chemistry. By now, the method has been tested in numerous clinical trials. 2024 saw the first approval of a CRISPR-Cas9-based gene therapy (Casgevy®) for the therapeutic treatment of sickle cell anemia and beta-thalassemia in the European Union.
However, the use of CRISPR-Cas9 is not without risk: If additional base pairs in the genome are altered erroneously, this can result in unintended mutations with potentially unpredictable consequences. This is particularly relevant in the therapeutic field. In response to this challenge, researchers have developed alternative technologies to minimize these off-target effects and increase the safety and specificity of therapeutic editing tools. This is where Frank Buchholz's ERC project DC-PGE comes in.
“The DC-PGE aims to help treat genetic diseases more safely and accurately by making precise and flexible changes to specific parts of the genome without undesirable side effects,” explains Frank Buchholz, PI of the project and Chair of Medical Systems Biology at TU Dresden's Faculty of Medicine as well as Head of Translational Research at the University Cancer Center Dresden (UCC).
Therapeutic tools that fulfill these characteristics are fully programmable DNA editing enzymes that can rearrange large DNA segments through recombination instead of just cutting them. As these can be used precisely and efficiently without the need to access intracellular signaling pathways, their application extends beyond monogenic diseases and expands the possibilities of gene therapy to an unprecedented level.
While there has been some success in engineering these enzymes to recognize and act on therapeutically relevant targets, their time-consuming and labor-intensive generation has hindered widespread implementation.
DC-PGE aims to address this shortcoming. "We have essentially improved a genome editing tool by adding a ‘safety lock’ mechanism. This mechanism ensures that the genome editing tool only becomes active if it has previously recognized the correct location in the DNA, making genome editing more accurate, as the tool only works where it is supposed to. This discovery enables us to develop highly efficient and, in particular, safer genome editing tools," Frank Buchholz explains the background.
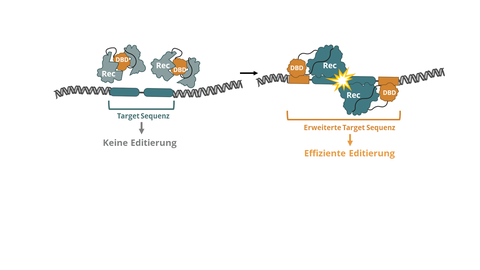
Model of a DNA-binding domain conditioned recombinase.
The aim of the Dresden scientists is to create a platform for the generation of fully programmable genome editing enzymes that do not rely on intracellular DNA repair mechanisms and that can directly and precisely target almost any genomic sequence. Moreover, their goal is to develop the designer-recombinase RecMECP2 to treat the MECP2 duplication syndrome, a rare genetic disorder that leads to severe symptoms such as immune system impairment, as proof of concept.
“This unique research approach combines different disciplines and cutting-edge technologies that have the potential to take the therapeutic possibilities of genome editing to the next level,” congratulates Prof. Esther Troost, Dean of the Faculty of Medicine. "In this project, the scientists along with Prof. Frank Buchholz are marking another major advance in this field. The ERC funding not only underlines the significance of this project and the excellence of the PI, but also the expertise at the Dresden science hub."
“Overall, DC-PGE will make a significant contribution to improving the quality of life and extending life expectancy by opening up new ways of treating and preventing diseases that were previously difficult to treat,” adds Prof. Uwe Platzbecker, Medical Director of the University Hospital.
About ERC Advanced Grants:
With the Advanced Grants, the European Research Council (ERC) supports experienced top scientists who have a track-record of significant research achievements over the last ten years and wish to develop new areas of research. Funding can be applied for for a period of up to five years with a maximum budget of EUR 2.5 million. In this funding round, a total of 2,534 applications were submitted, 281 of which received positive decisions. Throughout the application phase, Prof. Frank Buchholz and his team received active support from TU Dresden's European Project Center (EPC).
About Frank Buchholz:
Prof. Frank Buchholz has held the Chair of Medical Systems Biology at TU Dresden's Carl Gustav Carus Faculty of Medicine and has been Head of Translational Research at the University Cancer Center Dresden since 2010. Until 1997, Frank Buchholz worked as a doctoral student at the European Molecular Biology Laboratory (EMBL) in Heidelberg and did groundbreaking work in the field of genome engineering by developing sequence-specific recombinases and optimizing their use. During his postdoc phase at the University of California San Francisco (UCSF), he demonstrated for the first time that these enzymes can induce a predefined chromosomal translocation. He developed the substrate-linked directed evolution (SLiDE) technology to generate recombinases with new specificities. He perfected this scientific approach as group leader at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden from 2002 to 2010. In addition to his extraordinary scientific research, Frank Buchholz is one of Germany's most active academic founders in the life science sector and makes an essential contribution to transferring the results of basic research into innovative therapies.
Contact:
Anne-Stephanie Vetter
Staff Unit Public Relations of the Carl Gustav Carus Faculty of Medicine of TUD Dresden University of Technology
National Center for Tumor Diseases (NCT/UCC) Dresden
+49 351 458 17903
www.tu-dresden.de/med
