Jul 04, 2024
A closer look at cell toxins

Fluorescence micrograph of rat kidney cells after treatment with radioactive heavy metal ions.
Researchers examine how radionuclides interact with kidney cells
When radionuclides enter our organism, whether by inhalation, ingestion, or through wounds, they pose a potential health risk. Many previous studies on radionuclide exposure have focused mainly on animal experiments. However, we have little data on toxicity at the cellular and molecular level. Kidney cells are of particular interest because in mammals they play a central role in the detoxification of bivalent, trivalent, and hexavalent radionuclides as well as other heavy metals via urinary excretion. A team from the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and TUD – Dresden University of Technology has now found a differentiated picture, as the researchers report in the journal Science of the Total Environment (DOI: 10.1016/j.scitotenv.2024.171374).
Since radioactive heavy metals naturally occur in the earth’s crust, geological processes such as weathering and erosion can release them into water, soil, and air. Over the past 60 years, industrial, medical, and research use of radionuclides has also increased significantly. In particular, mining, accidents in nuclear power plants, and leaks in containment systems can release these elements into the environment. Another, albeit less significant, factor contributing to the release of radionuclides is their use in the diagnostics and therapy of cancer.
“Acute or chronic exposure to radioactive heavy metals and their radio- and chemotoxic effects poses a number of health risks to humans and animals. Once incorporated, they reach the kidneys via the bloodstream. Since the kidneys play a key role in excreting heavy metals, we are particularly interested in the interactions between these elements and renal cells,” says Dr. Astrid Barkleit from HZDR’s Institute of Resource Ecology, describing the focus of the research.
Previous studies have mainly focused on how such heavy metals accumulate in and are excreted from living humans and animals. By using mathematical biokinetic models, one can describe their general distribution in the body, but the underlying biological-chemical processes remain mostly unknown. The research team, therefore, investigated the effects of various heavy metals on renal cells and the chemical form in which the metal ions occur. These findings are essential for identifying suitable decorporation agents, i.e., complexing agents that remove accidentally incorporated metal ions from the body as gently as possible.
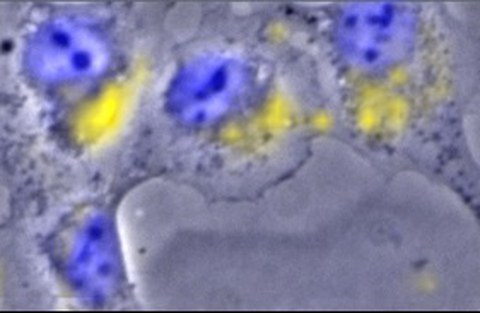
Distribution of europium(III) within a rat kidney cell, visualized for the first time using chemical microscopy, a unique coupling of Raman microscopy and luminescence spectroscopy. Blue: cell nuclei, yellow: europium.
“In our comprehensive study, we compared the effects of barium(II), europium(III), and uranium(VI) on human and rat kidney cells in vitro, i.e., under controlled laboratory conditions outside the living organism. The cell experiments were conducted at the Central Radionuclide Laboratory at TUD – Dresden University of Technology. Highly specialized analytical methods are available at both TUD and in the radiochemical controlled areas at HZDR. They enable us to use a unique combination of cell culture-based in vitro assays and microscopic, analytical, and spectroscopic methods. This allowed us to investigate cell viability, cell death mechanisms, and intracellular metal uptake of exposed cells. We were also able to determine the speciation of the heavy metals. Speciation describes the distribution of different chemical bonding forms of an element, in the cell culture medium and within the cells,” explains Dr. Anne Heller from the Chair of Radiochemistry/Radioecology at TUD. These findings help the scientists to understand the interactions of these heavy metals at the cellular and molecular level better.
Carefully selected line-up of heavy metals
The researchers chose the metal ions used in their study very thoughtfully. Barium(II) is suitable since it is a safe substitute for studying naturally occurring radioactive radium(II). Chemically speaking, non-radioactive europium(III) is very similar to the artificially produced radioactive elements americium(III) and curium(III). In addition, its excellent luminescence properties render it an ideal element for spectroscopic detection. As a naturally occurring heavy metal, easily soluble uranium(VI) is of particular importance for radioecology, especially here in Saxony, where the legacy of uranium mining, in particular the contamination of soil and water, is a major economic and technological challenge. In addition, a precise understanding of the complexation behavior of these heavy metal ions is of crucial importance both for general aspects of radiation protection and for the safety of planned radioactive waste repositories.
To investigate the chemical bonding form of heavy metals in the body, the scientists added aqueous metal solutions to the cells. As a result, the heavy metal ions lost their original water shell and became surrounded by the cellular molecules, so-called bioligands. Using special technologies, such as luminescence spectroscopy and chemical microscopy, the researchers were able to visualize the distribution of europium(III) in the cells. Fluorescence microscopy also revealed how the cells changed upon contact with the heavy metal: depending on the element, the cells swelled, their membrane fragmented, or parts of the cells detached.
This work is part of the joint project RADEKOR funded by the German Federal Ministry of Education and Research (BMBF) under grant numbers 02NUK057A and 02NUK057B.
Publication
C. Senwitz, D. Butscher, L. Holtmann, M. Vogel, R. Steudtner, B. Drobot, T. Stumpf, A. Barkleit, A. Heller, Effect of Ba(II), Eu(III), and U(VI) on rat NRK-52E and human HEK-293 kidney cells in vitro, in Science of the Total Environment, 2024 (DOI: 10.1016/j.scitotenv.2024.171374)
More information
Dr. Anne Heller
Chair of Radiochemistry/Radioecology
TU Dresden
Tel.: +49 351 463 32477
Christian Senwitz
Central Radionuclide Laboratory (ZNRL)
TU Dresden
Tel. +49 351 463 39219
Dr. Astrid Barkleit | Daniel Butscher
Institute of Resource Ecology at HZDR
Phone: +49 351 260 3136 | +49 351 260 3154
|
Media contact
Simon Schmitt | Head of Communications and Media Relations at HZDR
Phone: +49 351 260 3400 | Mobile: +49 175 874 2865 |
