Laura Meißner
Molecular motors from a 3D perspective:
motion and force generation of mitotic kinesins
Project: The aim of my project is to elucidate, how molecular motors work in cell division to organize the mitotic spindle.
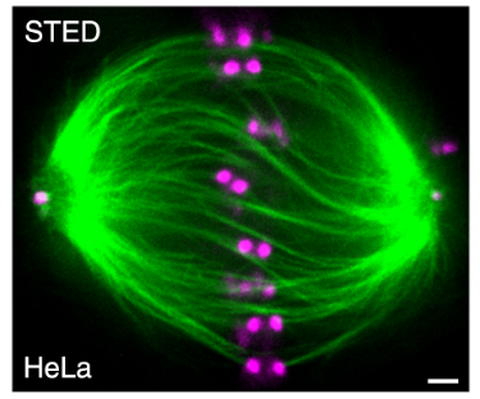
Figure 1: The mitotic spindle is twisted. Microtubule bundles in a metaphase spindle are twisted. Scale bar 1 µm. Modified from Novak et al. Nat. Comm. 2018.
During mitosis, the cell is using the spindle apparatus to distribute the duplicated chromosomes to the emerging daughter cells. Errors in this process can lead to chromosome instability and ultimately cancer. The spindle self assembles from microtubules, microtubule associated proteins and motor proteins, such as kinesin and dynein. Motors translocate along the microtubules, with the polarity of the microtubule orchestrating the direction of the motors. Some kinesins, such as kinesin-5 and kinesin-14, can bind two microtubules simultaneously, and in this way they are cross-linking and sliding them. Additionally, these two kinesins not only walk along the microtubule, but also step sideways (perpendicular to the long axis). This could lead to a rotation and twisting of microtubule bundles – which is observed in spindles of human cells, but the underlying molecular mechanism remains elusive
(Figure 1).
I am studying, how the antagonists human kinesin-5 (KIF11) and kinesin-14 (HSET) move along and slide microtubules and I am measuring their rotational forces to reveal, how they contribute to spindle organization.
Methods: To study the motion of motors, we are using a 3D sliding assay.
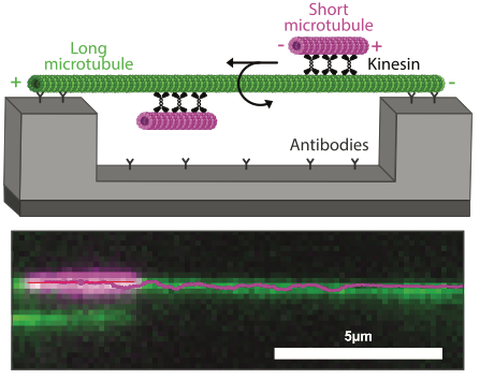
Figure 2: Kinesins drive the rotation of microtubules around each other. Schematics (top) and image with the track of a rotating microtubule (bottom) in a 3D sliding assay.
A long microtubule is suspended on ridge nanostructures, which allows the rotation of a short microtubule by motors (Figure 2). We image this sliding process with fluorescence microscopy and localize the microtubules precisely with a tracking software. The resulting tracks contain motility parameters, such as velocity, rotation frequency and motor spacing.
In the second part, we are measuring rotational forces in a coiling assay. Here, a microtubule is immobilized at one end, while motors drive the sliding and rotation
of the opposite end (Figure 3). Similar to turning a cord, the microtubule twists and snatches into a loop.
Using a theoretical model based on the Discrete Elastic Rods
Theory, we plan to calculate the forces from these microtubule bending shapes.
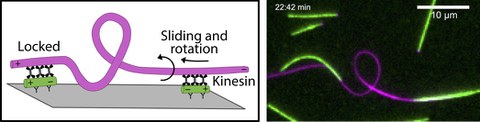
Figure 3: Kinesins generate rotational forces. Kinesins slide and rotate a microtubule, which coils and forms a loop.
By providing insights into the 3D behaviour of KIF11 and HSET, the project will contribute to understanding the biological functions of the motors – especially generation of the spindle twist.
2020
Kinesin-14 motors drive a right-handed helical motion of antiparallel microtubules around each other
A. Mitra, L. Meißner, R. Gandhimathi, R. Renger, F. Ruhnow, S. Diez
Nat. Commun. 11, 2565 (2020)
