Collective Effects in Motor Systems
Multi-Motor Unidirectional Transport
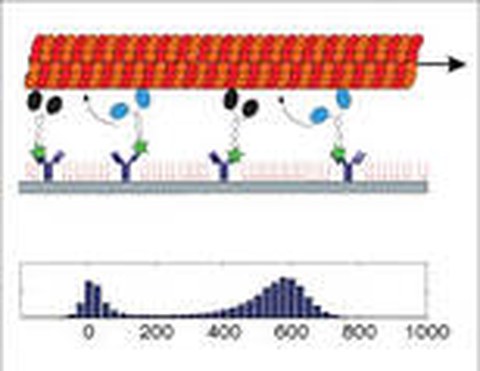
Do two or more motors that move the same cargo step in synchrony, producing the same step size as a single motor? In the case of transport by two kinesin-1 motors, we found successive 4-nm steps, corresponding to half the step size of a single motor. Along with results from dwell-time analysis this indicates that there is no coordination, in the sense of alternate stepping, between the motors (Leduc 2007). In addition, we asked how multimotor-based cargo transport is impaired if a fraction of the motors are defective. We found that impaired transport manifests in multiple motility regimes: (i) a fast-motility regime, (ii) a slow-motility regime, and (iii) a regime in which fast and slow motilities coexist. Notably, the transition from the fast to the slow regime occurred sharply at a threshold fraction of active motors (Scharrel 2014).
Multi-Motor Bidirectional Transport
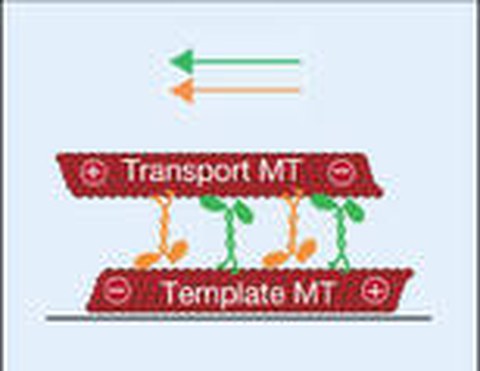
In many subcellular force-generating systems, groups of motor proteins act antagonistically. We are interested to study these systems in vitro by in reconstitution assays. For example, we studied the tug of war between superprocessive kinesin-1 motors acting on antiparallel microtubule doublets. In our experiments - as well as in our quantitative theory based on the physical properties of individual motors - we found distinct modes of slow and fast movements, as well as sharp transitions between these modes and regions of coexistence. Our results show that mechanical interactions between motors can collectively generate coexisting transport regimes with distinct velocities (Leduc 2010).
Transport by Membrane-Bound Motors
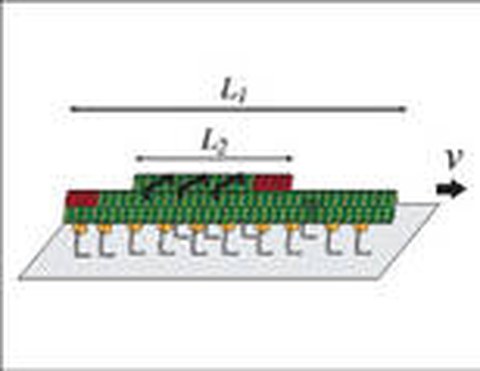
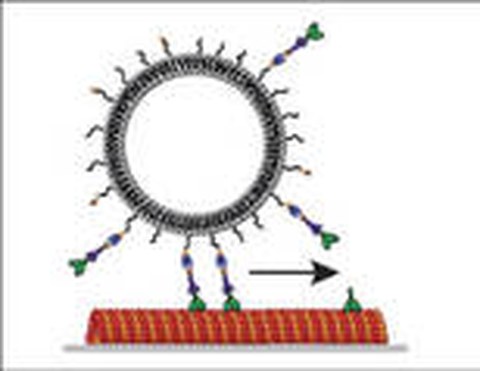
To mimic intracellular transport of membrane-bounded, vesicular cargo we anchored purified kinesin-1 motor proteins to the membrane of giant unilamellar vesicles, and studied their movement along two-dimensional microtubule networks. We found that the vesicles were transported by the cooperative activity of typically 5–10 motor molecules over distances up to the millimeter range, traveling with velocities identical to the velocities of cargo-free motors (Herold 2012).
Microtubule Crosslinking by Motors and MAPs
During mitosis and meiosis, the bipolar spindle facilitates chromosome segregation through microtubule-microtubule sliding. We showed that kinesin-14 alone can cause the sliding of anti-parallel microtubules but locks together those that are parallel. Single molecule imaging revealed that Ncd diffuses along microtubules in a tail-dependent manner and switches its orientation between sliding microtubules (Fink 2009). However, stable overlaps between ends of antiparallel microtubules - central elements within bipolar microtubule arrays - cannot be generated by molecular motors alone. We found that the presence of Ase1, a member of the conserved MAP65/PRC1 family of microtubule-bundling proteins, enables the formation of such stable overlaps through adaptive braking of kinesin-14-driven microtubule–microtubule sliding (Braun 2011).