Optical Technology Development
Total-Internal Reflection Fluorescence (TIRF) Microscopy
Fluorescence microscopy has become a powerful tool in cell biology. Developments over the past decade allow the efficient detection of individual fluorophores with the help of total-internal reflection fluorescence (TIRF) microscopy (Schlierf 2012). Fluorophores can be attached to molecular machines, e.g. motor proteins, and can be tracked with high resolution to study the chemo-mechanics of these enzymes and by extension yield insights into their cellular function. We use both, objective-type and prism-type TIRF microscopy to visualize the interaction of individual microtubule-associated proteins (MAPs). Technologically we developed new methods for convenient TIRF imaging as well as for calibrating the evanescent wave penetration depth (Gell 2009).
Fluorescence-Interference Contrast (FLIC) Microscopy
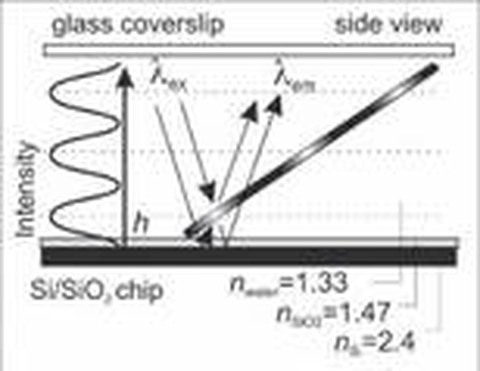
Fast and Spatially Resolved Environmental Probing Using Stimuli-Responsive Polymer Layers and Fluorescent NanocrystalsTo perform height measurements with nanometer precision, we use fluorescence-interference contrast (FLIC) microscopy, which is based on the self-interference of fluorescent light from objects near a reflecting surface. FLIC gives rise to a modulation of the detected intensity of a fluorescent object depending on its height above the surface. Utilizing an in situ calibration method, we used FLIC to determine that kinesin-1 holds its cargo about 20 nm away from the microtubule surface during active transport (Kerssemakers 2006). Moreover, we used FLIC microscopy to demonstrate that fluorescently-labeled, motile microtubules are versatile nano-probes to scan the geometry of engineered surfaces with nanometer height precision (Kerssemakers 2009) as well as to probe the conformation of stimuli-responsive polymer layers (Ionov 2006). This method, which is compatible with any kind of reflecting surface, permits dynamic and precise data acquisition in a highly parallel manner using a standard epi-fluorescence microscope. We currently employ FLIC microscopy to study the detailed 3D paths of motor proteins (labeled with quantum dots) on the surface of microtubules (Nitzsche 2008, Bormuth 2012).
Evanescent-Field Scattering Microscopy
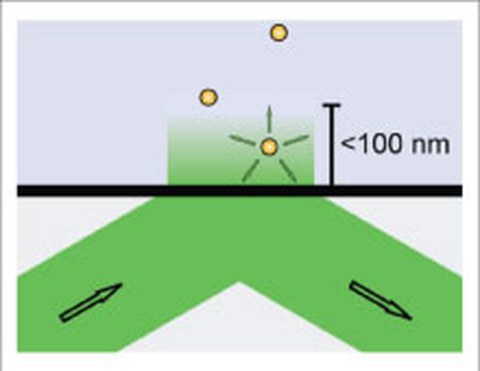
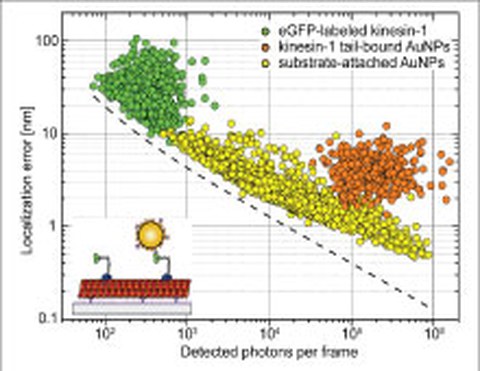
We developed a novel total-internal-reflection (TIR) approach based on a parabolically shaped quartz prism that allows for the detection of single-molecule fluorescence as well as single-particle scattering with high signal-to-noise ratios. We demonstrated homogeneous and spatially invariant illumination profiles in combination with a convenient control over a wide range of illumination angles and thus evanescent-field penetration depths. Evanescent-field scattering microscopy allows for sub-nanometer localization accuracies for the scattering of 40 nm gold nanoparticles (AuNPs) which - when bound to individual kinesin-1 motors - reliably report on the characteristic 8 nm stepping of kinesin-1 along microtubules (Schneider 2013). We currently apply this approach to study the interaction of individual motor proteins with obstacles on the surface of microtubules.
Image Processing with Nanometer Precision
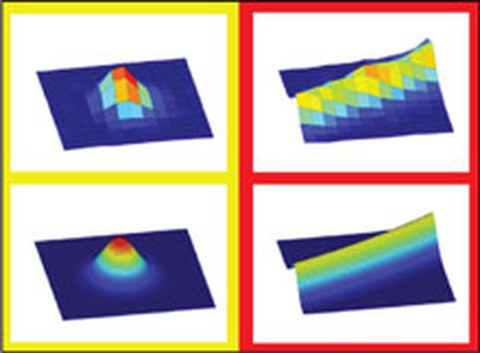
Recent developments in image processing have greatly advanced our understanding of biomolecular processes in vitro and in vivo. In particular, using Gaussian models to fit the intensity profiles of nanometer-sized objects have enabled their two-dimensional localization with a precision in the one-nanometer range. We develop algorithms to precisely localize single particles and curved filaments whose structures are characterized by subresolution diameters and micrometer lengths. Using surface-immobilized microtubules, fluorescently labeled with rhodamine, we demonstrated positional precisions of ~2 nm when determining the filament centerline and ~9 nm when localizing the filament tips. We currently apply these algorithms (i) to motor-proteins stepping on immobilized microtubules, (ii) to depolymerizing microtubules, and (iii) to microtubules gliding over motor-coated surfaces (Ruhnow 2011). The current version of our Fluorescence Image Evaluation Software for Tracking and Analysis (FIESTA) can be downloaded here.