Aug 29, 2016
From Rigid to Flexible - A physical mechanism to make the transport of cellular cargo efficient and specific
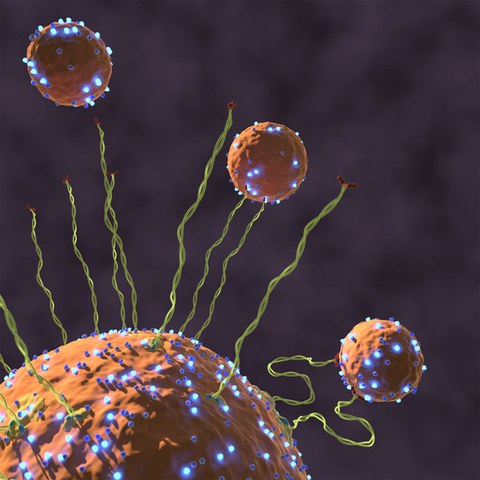
Capture of a vesicle by an endosome by the tethering factor EEA1 binding Rab5. Active Rab5 (shiny blue particles) induces a change in flexibility of EEA1 (green filaments) generating an entropic collapse force that pulls the vesicle toward the target membrane to dock and fuse.
In order for cells to function properly, cargo needs to be constantly transported from one point to another within the cell, like on a goods station. This cargo is located in or on intracellular membranes, called vesicles. These membranes have a signature, and only those with the correct signature may fuse with the membrane of another organelle into one compartment. The membrane itself must be recognized by a target membrane, which employs long tethering proteins to find its match.
David Murray and Marcus Jahnel from the labs of Marino Zerial at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) and Stephan Grill at the Biotechnology Center of the TU Dresden were curious to find out how these large tether proteins are able to recognize the signature of a membrane compartment and pull it in in order for the small fusion proteins to engage. They and their colleagues discovered that when the vesicle docks by an active protein called Rab5, GTPase, this protein is sending a message along the rigid tether protein to become flexible. This change in flexibility results in a force that starts the vesicle’s trip towards the target membrane to initiate docking and fusion. This newly found mechanism is published in the journal Nature and intuitively explains how traffic within the cell can be efficient and selective, and resolves a paradox of sizes.
Original Publication:
David H. Murray & Marcus Jahnel, Janelle Lauer, Mario J. Avellaneda, Nicolas Brouilly, Alice Cezanne, Hernán Morales-Navarrete, Enrico D. Perini, Charles Ferguson, Andrei N. Lupas, Yannis Kalaidzidis, Robert G. Parton, Stephan W. Grill and Marino Zerial: An endosomal tether undergoes an entropic collapse to bring vesicles together.
Nature, 24 August 2016, doi: 10.1038/nature19326
Further information: Group page Stephan Grill
