Jun 17, 2024
New rapid test platform for safe and fast respiratory diagnostics
Respiratory infections are among the greatest challenges facing healthcare systems worldwide. The faster and more reliably a diagnosis can be made, the sooner patients can be treated effectively and the burden on hospitals can be reduced. The validation project PL Analytix represents a decisive step toward a new generation of rapid tests. Funding from the European Union and the Free State of Saxony is making this possible.
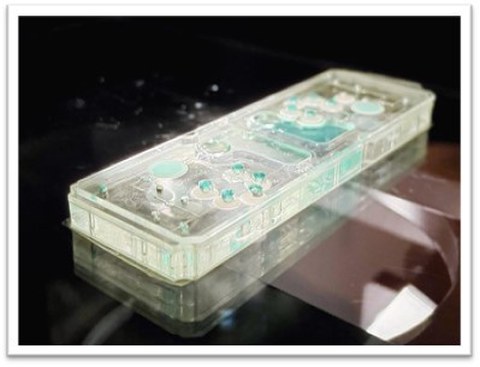
At the heart of the project is an innovative multiplex rapid test platform that combines the simplicity of conventional rapid tests with the high sensitivity and precision of PCR analyses.
A special feature of the platform are the smart material-based switching elements: tiny open and close valves that control the analysis process completely automatically – energy self-sufficient, resource-saving, and without an external energy supply. This makes the test not only precise, but also particularly robust and sustainable.
The platform's performance will be thoroughly tested as part of the project. At least 400 patient samples will be used to detect three different pathogens: influenza A/B, RSV, and SARS-CoV-2. The team follows the international ASSURED criteria for microfluidic systems, which set high standards for sensitivity, specificity, stability, and user-friendliness.
The results of the validation will form the basis for subsequent commercial exploitation. In addition to its application in respiratory diagnostics, other areas of application are opening up, for example in sepsis diagnostics or in the treatment of multi-resistant pathogens.
The project is making an important contribution to improving global healthcare. The combination of innovative diagnostics, sustainable technologies, and practical applicability shows how research can directly address the social challenges of our time.