Research overview
DNA and Chromosome Dynamics
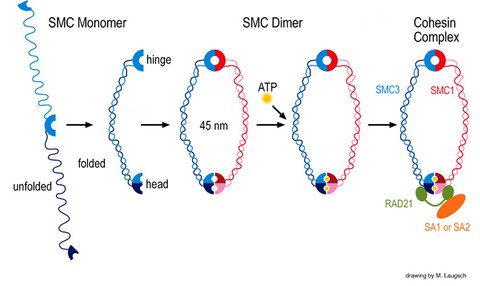
One of our projects aims at understanding the contribution of SMC (structural maintenance of chromosomes) proteins to DNA recombination and repair, sister chromatid cohesion, and related processes. The evolutionary highly conserved eukaryotic SMC protein family, with six members named SMC1 to SMC6, is involved in several key nuclear processes. The SMC family was defined as such in late 1994, and our publication in 1996 was the first on mammalian SMC proteins. Processes in which SMC proteins are involved are chromosome condensation, sister chromatid cohesion, DNA recombination and repair, in mitosis and meiosis. SMC proteins share a characteristic protein structure with coiled-coil-domains flanked by globular N- and C-terminal domains, and divided in the central region by a flexible hinge domain. The six types of SMC proteins form three types of heterodimers: SMC1 & SMC3, SMC2 & SMC4, and SMC5 & SMC6. All the heterodimers constitute core components of larger multiprotein complexes that carry out specific, ATP-driven functions in chromosome dynamics.
SMC Proteins and Cohesin
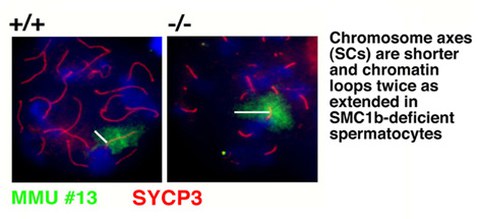
Our studies concentrate on the mammalian SMC1/3 heterodimer, which is a component of the sister chromatid cohesion complex cohesin. We have identified a meiosis-specific variant of SMC1, called SMC1ß , that is responsible for sister chromatid cohesion up to anapahase II of meiosis. Its role in meiosis and its potential failure in sterility and chromosome missegregation syndromes is currently being studied biochemically and in a variety of SMC1ß-deficient mouse models. SMC1ß-deficiency triggers strong meiotic phenotypes such as 50 % shortened chromosomal axes, and demonstrates that SMC1ß is essential for meiotic cohesion, synapsis, DNA recombination, and telomer behaviour.
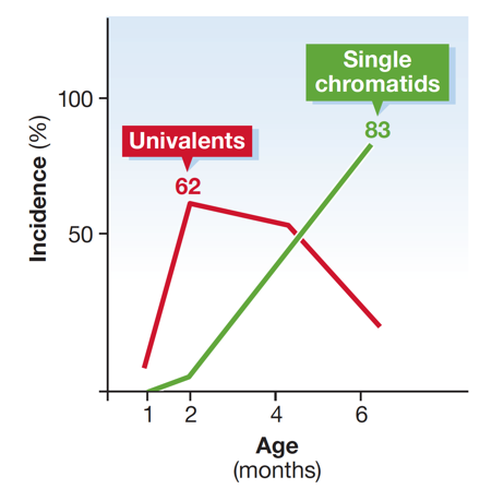
We demonstrated age-dependent increase in aneuploidies in SMC1ß-deficient oocytes, a unique model for human chromosome missegregation syndromes like trisomie 21. Recently, we have started to investigate the role of cohesin regulators in meiosis as they may be key to long-term function of cohesin in arrested oocytes but also for immediate roles in spermatocytes.
Increase of unpaired chromosomes and of single sister chromatids in SMC1ß-deficient murine oocytes with increasing age. This is not seen in wild-type oocytes. Loss of sister chromatid cohesion is a cause for chromosome missegregation and thus for aneuploidy.
A further addition to the research portfolio in this area is the analysis of a tudor domain protein, TDRD6, which plays important roles in miRNA regulation and chromatoid body formation in germ cells as a knockout mouse model, which we generated, showed. TDRD6 within the chromatoid body also controls nonsense-mediated decay in spermatids.
Recently, we embarked on testing the hypothesis that germ cell-specific genes, particularly meiotic genes, are involved in tumorigenesis in somatic tissues. Several lines of evidence support this hypothesis, which may lead to new avenues in tumor characterization and perhaps therapeutic approaches.
ACTIVATION OF HEMATOPOIETIC CELLS
Our second area of interest is in activation of cells like B cells, mast cells, osteoclasts and dendritic cells and signaling pathways that are involved in these processes. Currently, we focus on the properties and functions of a protein that we have identified, SWAP-70, and the similar DEF6/IBP.
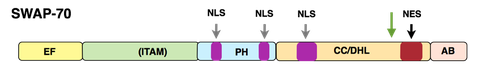
SWAP-70 structure
SWAP-70 was originally isolated by us from activated, mature murine B cells. SWAP-70 in a unique way combines features of signaling proteins, shuttles between cytoplasmic membrane, cytoplasm and nucleus in activated B cells, but in other cells is found only at the cytoplasmic membrane and in the cytoplasm. In B cells, SWAP-70 is involved in immunoglobulin class switch recombination, in particular the switch to the IgE isotype, the "allergy isotype". We have characterized SWAP-70 as an unusual type of PIP3-binding, Ras-independent, Rac-interacting, F-actin binding protein, which through its pleckstrin homology domain (PH) associates with the cytoplasmic membrane and localizes to F-actin structures. SWAP-70 bundles F-actin and prevents depolymerization of the filaments; it also binds and inhibits cofilin, an F-actin severing protein.
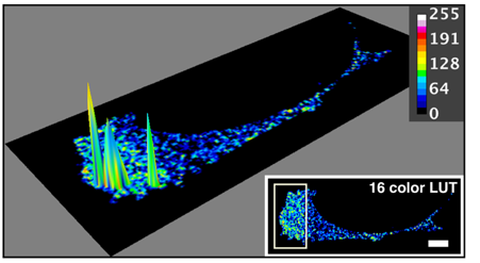
Colocalization of SWAP-70 and cofilin in an activated cell, measured by FRET
Our SWAP-70 deficient mice display several specific phenotypes such as deficiencies in CD40-signaling in B cells including the switch to IgE, impairment of mast cell degranulation, failure to form actin rings in osteoclasts, deficiencies in membrane actin rearrangements in a variety of cells, an autoimmune phenotype, and elevated apoptosis in response to DNA damage.

Model of SWAP-70's role as a promoter driving IgE production
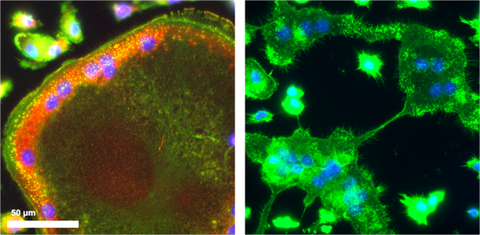
Absence of F-actin rings in SWAP-70 deficient osteoclasts, which also fail to fuse (right); SWAP-70 (red); F-actin (green)