COMBAT-PDAC: Personalized Treatment for Pancreatic Cancer
Pancreatic ductal adenocarcinoma (PDAC) is projected to become the second leading cause of cancer-related deaths in Europe by 2040. Unlike many other cancers, PDAC survival rates have remained alarmingly low for over 50 years, with a median survival of just 8–10 months after diagnosis. A key challenge lies in the high degree of genetic and non-genetic heterogeneity within individual tumors—causing unpredictable disease progression and poor treatment responses. Yet, current diagnostic and therapeutic strategies fail to account for this complexity.
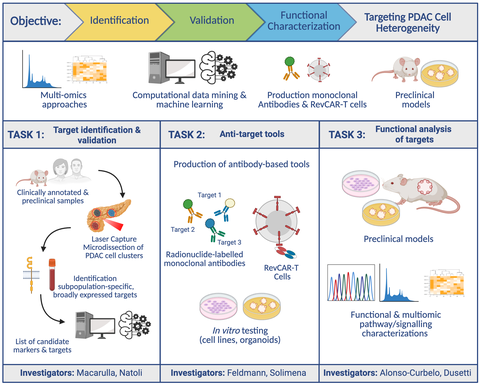
The COMBAT-PDAC project is a three-year international research initiative uniting experts from Germany, France, Italy, and Spain. Our goal is to develop personalized therapeutic strategies that target the full spectrum of PDAC cell populations. Preliminary research from members of our consortium identified promising cell-surface biomarkers and therapeutic targets that emerge during PDAC progression. Building on this foundation, the project is structured into three core scientific work packages (WPs), supported by a fourth coordination WP.
Approach & Key Objectives
WP1: Identifying and validating combinatorial biomarkers and therapeutic targets (Macarulla|Natoli)
COMBAT-PDAC will use molecular profiling techniques, including spatial transcriptomics, single-molecule fluorescence in situ hybridization (smFISH), and multiplex immunofluorescence, to map PDAC heterogeneity with extremely high resolution. Machine learning will be applied to identify the most effective biomarker-target combinations for diagnosis and treatment.
WP2: Engineering monoclonal antibody-based tools and adaptable CAR-T cell therapies (Feldmann|Solimena)
To overcome PDAC’s resistance to conventional therapies, COMBAT-PDAC will develop a flexible and multi-targeted immunotherapy approach. The project will develop and characterize monoclonal antibodies (mAbs) and RevCAR-T cells—an innovative, switchable CAR-T cell technology allowing for precise, combinatorial tumor targeting. These tools will be validated in preclinical models.
WP3: Mechanistic insights into PDAC progression and therapeutic impact (Alonso-Curbelo|Dusetti)
Using functional genomics, advanced in vivo models, and synthetic lethality studies, WP3 will investigate how the identified targets drive PDAC progression. The effectiveness of mAbs, RevCAR-T cells, and antibody derivatives will be tested in clinically relevant models.
Clinical & Translational Impact
COMBAT-PDAC is designed with a clear translational pathway—bridging cutting-edge research with real-world clinical application. This project will:
-
Enable earlier and more precise PDAC detection through innovative theranostic biomarkers.
-
Develop multi-targeted therapies that adapt to tumor heterogeneity, improving patient outcomes.
-
Lay the foundation for future clinical trials, with industrial partnerships for GMP production.
-
Engage with regulatory agencies and patient organizations to facilitate real-world implementation.
A Step Toward the Future of Cancer Treatment
While PDAC is our main focus, the impact of COMBAT-PDAC may extend to other aggressive, heterogeneous cancers such as lung cancer. By integrating molecular profiling, machine learning, and adaptable immunotherapy, this project could redefine how we diagnose and treat some of the deadliest cancers. Through our collaborative, multidisciplinary efforts, COMBAT-PDAC aims to turn scientific innovation into personalized, life-saving treatment strategies for patients worldwide.
Collaborators
COMBAT-PDAC has collaborators from leading institutions across Europe.
Coordinator
Michele Solimena - Germany - TU Dresden, Department of Molecular Diabetology. Funded by the Saxon State Ministry for Science, Culture and Tourism (SMWK).
Partners
Nelson Dusetti - France - Cancer Research Center of Marseille, Translational Research and Innovative Therapies. Funded by the French National Research Agency (ANR).
Anja Feldmann - Germany - Helmholtz-Zentrum Dresden-Rossendorf e.V., Institute of Radiopharmaceutical Cancer Research, Department of Radioimmunology. Funded by the Saxon State Ministry for Science, Culture and Tourism (SMWK).
Gioacchino Natoli - Italy - Istituto Europeo di Oncologia, Department of Experimental Oncology. Funded by the Italian Ministry of Health (IT-MoH).
Direna Alonso-Curbelo - Spain - Fundació Institut de Recerca Biomèdica, Cancer Science Programme. Funded by the Departament de Salut-Generalitat de Catalunya (DS-CAT).
Teresa Macarulla - Spain - Vall d’Hebron Institute of Oncology (VHIO), Upper Gastrointestinal Cancer Translational Research Group. Funded by Carlos III Health Institute (ISCIII).
The European Union offers a variety of funding programmes to support research and education as a valuable public good. Our project was funded by the European Regional Development Fund (ERDF).