Nov 22, 2024
Newly funded “EP Permed” project targets pancreatic cancer diversity for better diagnosis and treatment
Pancreatic ductal adenocarcinoma (PDAC) is expected to become the second-leading cause of cancer-related deaths in Europe by 2040. Unlike other types of cancer, survival rates for pancreatic cancer have barely improved over the past 50 years, and the typical patient lives only 8–10 months after diagnosis. This is mainly due to the lack of actionable combinatorial biomarkers and theranostic agents targeting the non-genetic heterogeneity in PDAC. Now, an international research consortium, under the coordination of Michele Solimena of the Paul Langerhans Institute Dresden, will tackle these issues.
In parallel to researchers from the PLID, the multidisciplinary and international project team includes experts from research institutions such as the Helmholtz-Zentrum Dresden-Rossendorf (HZDR), the European Institute of Oncology (IEO) in Milan, the Institute for Research and Biomedicine (IRB), the Vall d'Hebron Institute of Oncology (VHIO) in Barcelona and the Marseille Cancer Research Center (INSERM U.1068). The team will receive almost two million euros through the European Partnership for Personalized Medicine (EP PerMed) for the “COMBAT-PDAC” project, which aims to develop and validate combinatorial strategies to target phenotypically distinct PDAC cell populations in each tumor.
Pancreatic cancer is a highly aggressive and deadly disease, with a median survival rate of less than 12 months after diagnosis. Despite significant advances in medical research and treatment, the survival rate for PDAC patients has not improved significantly over the past 50 years. One of the main reasons for this is the high level of genetic diversity within tumors, which makes it difficult to develop effective treatments. This diversity is characterized by the presence of multiple cell populations with distinct genetic and molecular profiles, which can lead to treatment resistance and disease progression. Researchers have attempted to classify PDAC tumors based on their molecular characteristics, but these systems have limitations and do not account for the tumor's natural diversity. “For example, many classification systems rely on bulk transcriptomic data, which can mask the underlying heterogeneity of the tumor. As a result, these systems have limited clinical impact and do not provide a comprehensive understanding of the tumor's biology”, said Prof. Solimena. “To improve treatment outcomes, it's essential to develop new approaches as well as biomarkers that can accurately capture and address the tumor's intrinsic heterogeneity. PDAC is a frequent comorbidity of diabetes. Intriguingly, our work on the biology of pancreatic beta cells led us to serendipitously identify a potential novel biomarker and target for PDAC detection and therapy.”
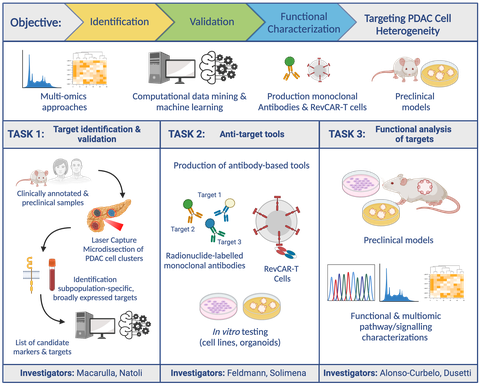
In the next three years, the COMBAT-PDAC project aims to address this gap by focusing on understanding and targeting the whole spectrum of heterogeneous pancreatic cancer cells present in individual tumors. By combining several advanced methods, such as in-depth genetic analysis, data-driven insights, and innovative therapies including engineered antibodies and engineered T lymphocytes (CAR-T cells), the COMBAT-PDAC scientists seeks to find ways to effectively target the many types of cells within a single tumor. The project’s ambiguous goals include identifying and validating specific markers that can detect the diverse cell types in pancreatic tumors, creating and testing tools to specifically target these different cells, and understanding how these markers influence the progression of the disease. This comprehensive approach could reveal new strategies for diagnosis and pave the way for personalized treatment options.
“Through these efforts, our COMBAT-PDAC team hopes to develop groundbreaking tools that better address the complexity of pancreatic cancer, potentially improving outcomes and survival for patients”, concludes Solimena.
The new COMBAT-PDAC project emerged from a smaller seed project funded by the University Hospital, the Carl Gustav Carus Faculty of Medicine at TU Dresden and the Helmholtz-Zentrum Dresden-Rossendorf and which was intended to strengthen the link between metabolic and cancer research.