Forschung
Project 1: Dynamics of Glycosphingolipids and Gangliosides: Insights into Membrane Interactions and Cancer Pathogenesis
Background: Glycosphingolipids (GSLs) are essential constituents of cell membranes playing a significant role in a wide range of biological processes such as recognition of hormones, bacterial and viral toxins, cell growth and differentiation, cell-cell interaction and signaling. Their pivotal roles make them key targets for biomedical research and therapeutic interventions. Aberrant glycan epitopes are a classic hallmark of malignant transformation, yet their full clinical potential in cancer diagnostics and therapeutics is yet to be realized. This is partly because our understanding of how the accessibility of these epitopes to the immune system is regulated remains poorly understood.
A good example is the increased level of Neu5Gc, a non-endogenously produced sialic acid which can be acquired from a dietary source such as red meat and dairy products. Interestingly, Neu5Gc can be recognized as a foreign molecule by the human immune system, thus its incorporation into glycolipids and glycoproteins can potentially be leading to chronic inflammation, which is implicated in various diseases, including cancer.
Aims: The goal of the project is to uncover the molecular mechanisms through which the gangliosides containing Neu5Gc could be utilized for targeting.
Project 2: Lipid-mediated regulation of EGFR in cancer biology
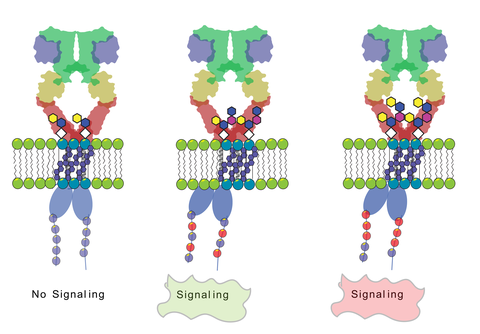
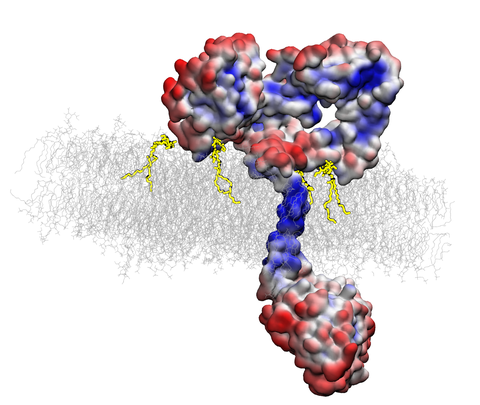
Background: The human Epidermal Growth Factor Receptor (EGFR) is a key receptor tyrosine kinase involved in regulating cellular processes such as growth, differentiation, and survival. EGFR signaling pathways are frequently dysregulated in many types of cancer (malignancies), thus it remains a significant target for anti-neoplastic treatments such as chemotherapy, immunotherapy, etc. Dysregulation of EGFR can occur through various mechanisms, such as receptor overexpression, gain of function mutations, or increased production of EGFR ligands. Also, lipids can directly or indirectly modulate EGFR signaling by affecting its activity, trafficking, turnover, localization, and downstream signaling.
We have previously demonstrated that the lipid environement around EGFR plays an important role in prohibiting EGFR from activation in an uncontrolled ligand-free manner. We have also shown that GM3 glycolipid prevents the phosphorylation of the EGFR without affecting its ligand-binding properties. The above discussed biochemical observations raise an intriguing question as to how the conformational changes in EGFR are induced by the lipid environment surrounding the receptor.
Aims: 1) Uncover how specific lipid compositions within the cell membrane induce conformational changes in EGFR; 2) Investigate the impact of lipidomic changes during tumorigenesis on EGFR signaling; 3) Explore the molecular mechanisms by which the membrane's physicochemical properties modulate EGFR activity.