Development and demonstration of Sodium-Sulfur battery systems
Duration: July 2016 - June 2018
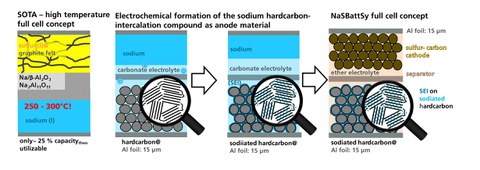
Renewable energy types, such as wind and photovoltaic energies, are subject to natural variations in quantity. To compensate for these variations, high-temperature sodium-sulfur batteries are employed, for example, as stationary energy cells. Because of the kinetic mechanism of the reaction, only less than 25 percent of the theoretical capacity can be utilized. Furthermore, the high-temperature system has tremendous safety risks due to the use of liquid sodium.
Dresden research institutes are collaborating in the funded project on novel battery components and manufacturing techniques for a room temperature Na-S battery demonstrator. The development tasks include material preparation, electrode production, and formation of cell stacks. In the joint NaSBattSy project, registered under No. 100234957 by the SAB (Saxon Development Bank), the consortium sought to develop a stationary electrochemical energy cell based on sodium-sulfur cell technology. This mode of energy storage can be produced at reasonable costs and works at room temperature.
An innovative, electrochemical step is required, in which the anode material – a sodium-carbon compound NaxC – is generated. Customized electrolyte formulation must efficiently eliminate undesirable side effects (polysulfide shuttle) and form protective layers on the anode side. For the anode, non-graphitizable carbons are used; these carbons are presodiated with an adapted electrolyte formulation and simultaneously coated with a sulfidic protective layer.
In the collaboration, the project partners created the electrochemical and technological basics for a new electric storage system for stationary applications. For the first time, a Na-S cell could demonstrate electrochemical stability for more than 1000 cycles at room temperature. This result is an important milestone towards low-cost energy cells based on available raw materials. In the future, cyclic life can be further prolonged by adapting the electrolyte formulation.