Aug 04, 2020
Go with the counter-rotating flow!
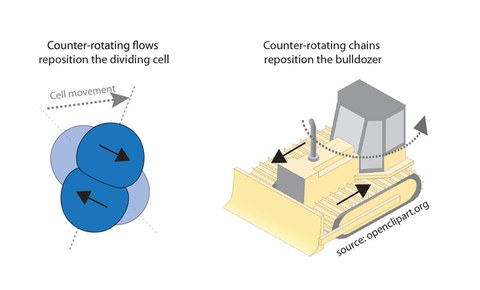
Left: Schematic of a symmetrically dividing (AB - lineage) cell (top view) undergoing repositioning during cell division, Copyright: Lokesh Pimpale et al.; right: Schematic of a rotating bulldozer (side view), Copyright: source: openclipart.org by danjiro. Solid back arrows indicate the direction of counter-rotating flows (left picture) and counter-rotating chains (right picture) and dotted arrow represents the direction of movement.
Every living organism grows from one single cell. During development, the one cell embryo undergoes numerous rounds of cell division to generate a fully functional organism. The embryo needs to position the newly dividing cells correctly to ensure that they are in contact with the right neighbor cells and receive the right signals. Only then they can properly develop and differentiate further.
The cell cortex - a layer of actin fibers and myosin motor proteins just below the cell membrane – is known to play an important role in cell positioning. The interaction of actin and myosin generates “tug-of-war like” forces which give rise to flows of the cell cortex. These flows in turn can reposition cells as they divide to form two daughter cells. However, prior to this study it was not clear how common cells are repositioned by these rotating flows.
Researchers from the group of Stephan Grill, director at the MPI-CBG, and affiliated to the Center for Systems Biology Dresden, the Cluster of Excellence 'Physics of Life' of the TU Dresden and the Biotechnology Center of the TU Dresden have now found that counter-rotating flows in the cortex of the Caenorhabditis elegans roundworm drive movement of specific, but not of all cells during development. In their work just published in eLife the scientists show that counter-rotating flows are only observed in cells undergoing symmetric cell divisions.
How can counter-rotating flows of the cell cortex reposition cells? Lokesh Pimpale, PhD student in the Grill lab, and first author of the study, explains, “Counter-rotating flow of the cell cortex turn a cell much like a bulldozer turns on the spot, by rotating both of its chains in opposite direction. We have discovered that the occurrence of counter-rotating flows is cell-lineage specific, meaning that only cells of the AB-lineage of the nematode worm undergoes counter-rotating flows and cell skews.”
By tracking cell positions the researchers then showed that counter-rotating flows drive the movement of symmetric cells throughout all stages of cell divisions and always results in a skewed cell arrangement. Stephan Grill, who supervised the work, adds “The actomyosin network generates the forces that drive development, and it can generate rotating movements of cells. We here show that these rotating movements and the resultant cell skews are much more prevalent than perhaps anticipated, and that they are under precise developmental control. This work is exciting because it reveals that intricate physical forces such as torques generated by counter-rotating flows are at work when an organism is shaped.”
Publication:
Lokesh G Pimpale, Teije C Middelkoop, Alexander Mietke, Stephan W Grill: „Cell lineage-dependent chiral actomyosin flows drive cellular rearrangements in early C. elegans development“, eLife, 9 July, 2020, doi: 10.7554/eLife.54930
