Jun 24, 2024
Translation Factors in the Hot Seat: How Cells Regulate Protein Production During Heat Shock
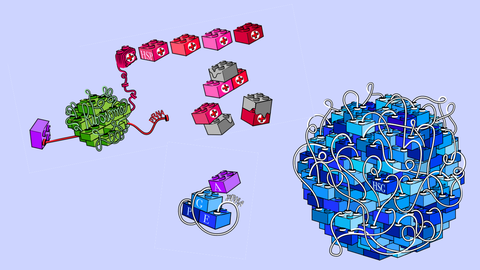
An artistic representation of the study results. Using bottom-up biochemistry and cellular experiments, the Alberti group rebuilt eIF4F complex from individual blocks and found that heat inactivates it.
A sudden temperature rise alters the three-dimensional structure of proteins and impairs their function within cells. In response to heat shock, cells immediately react by shutting down the production of proteins and producing “first-aid” proteins instead. However, the mechanism behind this rapid response is not well understood. Researchers from the Alberti group at the Biotechnology Center (BIOTEC) of Dresden University of Technology identified translation factors that regulate the production of proteins upon heat shock. The findings were published in the journal Molecular Cell.
Proteins rely on their unique three-dimensional (3D) structures to function. Such 3D structure is sensitive to elevated temperatures that can cause proteins to misfold and lose function. To survive, cells implement a series of protective measures. They stop producing new proteins and turn on the production of “first-aid” proteins, so-called molecular chaperones that rescue damaged proteins. It has long been known that this response coincides with the assembly of special cellular compartments known as heat shock granules. The function of heat shock granules during heat shock though had remained largely unknown.
“Our understanding of how cells react to environmental stress traditionally focused on transcription regulation. But it was also clear that the translation machinery which produces the proteins for the cell also changes its activity during heat shock. Yet the molecular events and details are still not clear. We now show that key translation factors assemble into these heat shock granules to regulate protein production” says Prof. Simon Alberti, research group leader at Biotechnology Center (BIOTEC) who led the study.
eIF4G: A Molecular Shapeshifter
The Alberti team used a bottom-up reconstitution approach, recreating protein production upon heat shock in the test tube. “We purified components needed for protein production such as translation factors and mRNA. This allowed us to study the heat shock response without the complexity of a living cell,” explains Christine Desroches Altamirano, the main author behind the study. “Importantly, once we got a mechanistic understanding, we could go back to cells and validate our findings.”
The central player of this story – eIF4G – acts like a docking station, coordinating a precise assembly of multiple translation factors on messenger RNA (mRNA) at sites of protein production. Upon heat shock, eIF4G ‘shapeshifts’ and promotes the assembly of ‘arrested’ complexes and heat shock granules. Crucially, these complexes inhibit protein production.
“The structural rearrangement, or shapeshift, of the eIF4G protein is a robust mechanism. This demonstrates that cells can ‘sense’ temperature changes directly at the level of protein conformations,” explains Titus Franzmann, a senior scientist in the Alberti lab.
Translation Factors in the Hot Seat
Interestingly, while eIF4G assembles into heat shock granules with multiple translation factors to repress protein production, another translation factor – eIF4A – escapes interaction with eIF4G and aids in the production of “first-aid” proteins.
“Our findings show that translation factors play a crucial role in regulating protein production during heat shock. We identify two types of translation factors: first, there are the shapeshifters like eIF4G which repress protein production, and second, there are translation factors like eIF4A that promote the production of “first-aid” proteins. We now have a new level of understanding about how changes in the cellular environment, such as temperature, can be detected at the molecular level,” concludes Prof. Alberti.
Original Publication
Christine Desroches Altamirano, Moo-Koo Kang, Mareike A. Jordan, Tom Borianne, Irem Dilmen, Maren Gnädig, Alexander von Appen, Alf Honigmann, Titus M. Franzmann, Simon Alberti: eIF4F is a thermo-sensing regulatory node in the translational heat shock response. Molecular Cell (May 2024)
Link: https://doi.org/10.1016/j.molcel.2024.02.038
