May 04, 2022
With or Without Sleep: Sleep Neuron Activity Boosts Protective Gene Expression and Safeguards Survival
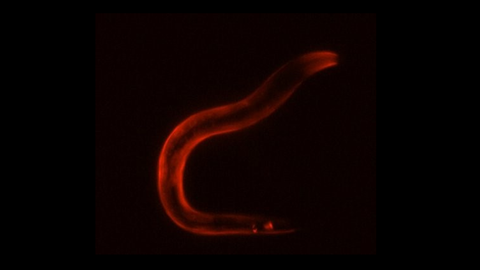
Activation of the sleep neuron causes a stress gene expression response in the entire body of the worm, visualized here in red by staining for HSP-12.6, a Heat Shock Protein required for survival.
Using the C. elegans worm, Dresden scientists show that sleep neuron activity controls protective gene expression during sleep. Disturbing sleep leads to overactivation of the sleep neuron, further promoting the protective gene expression.
Sleep refreshes the brain and the body by inducing protective changes in gene expression. Sleep disturbance triggers a stress response that also turns on protective genes. It was unclear how sleep or sleep deprivation can lead to these changes in gene expression. Scientists led by Prof. Henrik Bringmann from the Biotechnology Center (BIOTEC) of TU Dresden used the C. elegans worm to show that the protective gene expression during sleep is controlled by the activity of sleep neuron and that during sleep deprivation, overactivation of sleep neuron boosts this safeguarding process. Their results present a potential paradigm shift in understanding the consequences of sleep loss on gene expression. The study was published in the journal Current Biology on May 2.
Sleep is an essential process that influences all tissues and systems in our bodies. On a molecular level, sleep induces the expression of genes that help maintain the brain and body. Missing a night of sleep is a tremendous challenge to the body. It activates genes that carry out stress response and protect the body from the consequences of sleep deprivation. Part of this stress response is activation of protective genes from the so-called FOXO pathway. This pathway is involved in a multitude of cellular processes that overall contribute to recovery, survival, and longevity. How sleep and lack of sleep trigger these changes in gene expression was not understood. To address this long-standing question, scientists at the Biotechnology Center (BIOTEC) of TU Dresden led by Prof. Henrik Bringmann studied sleep in C. elegans worms.
“To trigger sleep, our body has to turn off wakefulness. There is a special set of sleep neurons for this task. These sleep neurons send signals that shut down other neurons responsible for arousal, and in this way promote sleep. Humans have thousands of these sleep neurons located in various centers in the brain,” says Prof. Henrik Bringmann. “What makes C. elegans a wonderful minimal model to study sleep is that it has only one key sleep-active neuron that induces sleep.”
It’s All About the Sleep Neuron
The Bringmann team wanted to test how this sleep neuron affects changes in gene expression during sleep. “Sleep neurons are active during sleep, and they are activated even further during sleep deprivation. This might be counterintuitive at first but it is because our body acts as a homeostat. If something throws it off balance, our body tries to compensate to restore equilibrium. In this case, disturbing sleep causes the body to activate sleep neurons more and more, in an attempt to force sleep,” explains Prof. Bringmann.
The team has genetically engineered two versions of the C. elegans worms. One type had its sleep neuron permanently inactive and the other permanently active. “Both of these extreme situations resulted in the loss of sleep. This was an experimental advantage for us, as we were able to test whether the sleep neuron controls gene expression independently of sleep,” says Prof. Bringmann.
As a result, scientists observed that the expression of stress response and protective genes decreased when sleep neuron was off. On the other hand, the expression of these genes increased when the sleep neuron was permanently active. “These results show that the protective gene expression is a function of sleep neuron activity,” explains Prof. Bringmann.
The results provide a new interpretation of the consequences of disturbing sleep in C. elegans. ”Our experiments suggest that the protective gene expression response that is observed when sleep is disturbed is rather not caused by the actual loss of sleep, but by the overactivation of the sleep neuron,” says Prof. Bringmann.
Lessons From The Worm
The results provide an unexpected link between sleep neuron activity and gene expression. While the results originate from the C. elegans worm, they present a potential paradigm shift for understanding the consequences of sleep deprivation and insomnia also in other animals.
“Disturbing sleep is known to cause overactivation of sleep-active neurons in many animals,” adds Prof. Bringmann. “It could be that the activity of sleep neurons controls stress response and the protective, longevity-related gene expression also in other animals and perhaps even in humans. These questions make for an interesting topic of further studies.”
Original Publication
Anastasios Koutsoumparis, Luisa M.Welp, Alexander Wulf, Henning Urlaub, David Meierhofer, Stefan Börno, Bernd Timmermann, InkaBusac, Henrik Bringmann: Sleep neuron depolarization promotes protective gene expression changes and FOXO activation. Current Biology (May 2022)
Link: https://doi.org/10.1016/j.cub.2022.04.012
About the Biotechnology Center (BIOTEC)
The Biotechnology Center (BIOTEC) was founded in 2000 as a central scientific unit of the TU Dresden with the goal of combining modern approaches in molecular and cell biology with the traditionally strong engineering in Dresden. Since 2016, the BIOTEC is part of the central scientific unit “Center for Molecular and Cellular Bioengineering” (CMCB) of the TU Dresden. The BIOTEC is fostering developments in research and teaching within the Molecular Bioengineering research field and combines approaches in cell biology, biophysics and bioinformatics. It plays a central role within the research priority area Health Sciences, Biomedicine and Bioengineering of the TU Dresden.
www.tu-dresden.de/cmcb/biotec
www.tu-dresden.de/cmcb
Scientific Contact:
Prof. Henrik Bringmann
Biotechnology Center (BIOTEC) of TU Dresden
Tel: +49 (0) 351 463 40330
Email:
Webpage: https://tud.link/uxep
