Forschung Dr. Torben Mentrup
Aspartic intramembrane proteases
Beyond the characterisation of novel substrates of SPP/SPPL proteases in various pathophysiologically relevant systems driven by the Schröder lab, the fascinating family of aspartic intramembrane proteases (I-CLIPs) is also the main focus of our subgroup. In our research we focus on two aspects of the biology of these fascinating enzymes.
Our first central research branch is the analysis of aspartyl I-CLIPs in the context of male fertility. Gametogenesis and gamete function are highly complex processes which despite intensive research are still only poorly understood. This is also exemplified by the role of aspartyl I-CLIPs in this field of biology. Even though all these enzymes are expressed within the murine testis, their role is still largely enigmatic. We have obtained unexpected insights into the function of aspartyl I-CLIPs in this organ by the characterisation of a novel interactor of the testis-specific SPPL2c protease which we have named Frey1. Frey1 is a 99 amino acids long type II protein that is specifically expressed in round spermatids within the murine testis. Here, Frey1 is stabilised by binding to the catalytic centre of the protease which aids its role as chaperone for the formation of Izumo1-containing complexes that are essential for sperm-oocyte fusion. Based on this function, Frey1-deficient male mice suffer from normozoospermic infertility (Contreras et al., 2022). In future and already initialised projects we will utilise the established methodological pipeline to decipher the role of the other aspartic I-CLIPs and their regulators in gametogenesis as well as male fertility.
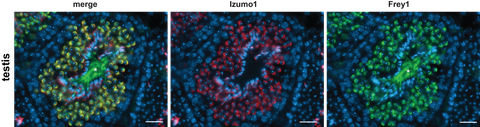
Expression of Frey (green) and Izumo1 (red) in the murine testis. Nuclei are stained with DAPI. Scale bar, 25 µm.
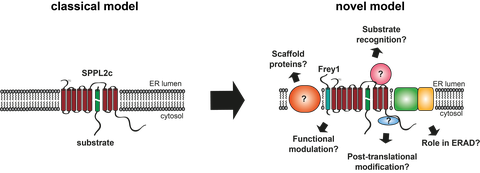
The second aim of our subgroup is to uncover regulatory pathways that impact on the activity of aspartic I-CLIPs. In the classical model of intramembrane proteolysis, these enzymes were believed to function in a rather unregulated manner as a kind of “proteasome of the membrane”. While this has been proven wrong for the closely related γ-Secretase which is a key proteolytic complex involved in the development and Alzheimer’s disease and Notch signalling, for a long time SPPL2 proteases were still thought to function in mono- or homomultimeric proteins. By contrast, we could demonstrate that binding of Frey1 to the catalytic centre of SPPL2c at least in overexpression systems interferes with the enzymatic activity of the enzyme establishing Frey1 as the first protein inhibitor of an aspartic I-CLIP in general. Even though the observed inhibitory action of Frey1 is highly specific for SPPL2c, we identified an inhibitory motif that could be utilised to generate substrate-based inhibitors of the γ-Secretase demonstrating the broad applicability of the identified motif (Contreras et al., 2023). Based on the identification of Frey1 and first non-catalytic functions of the SPPL2c protease we believe that regulation of SPPL2 proteases is indeed much more complex than initially anticipated (see Figure below). Therefore, understanding of the physiological functions of these enzymes requires a much deeper understanding of the interactome of these enzymes, which we aim to further characterise in already initiated and future research projects.
Recent Publications:
1. Contreras W, Wiesehöfer C, Schreier D et al. (2022) C11orf94/Frey is a key regulator for male fertility by controlling Izumo1 complex assembly. Science advances 8(32):eabo6049. doi: 10.1126/sciadv.abo6049
2. Contreras W, Bazan JF, Mentrup T (2023) The transmembrane domain of Frey1 harbors a transplantable inhibitory motif for intramembrane proteases. Cell. Mol. Life Sci. 80(6):170. doi: 10.1007/s00018-023-04823-7